|
Cytomegalovirus
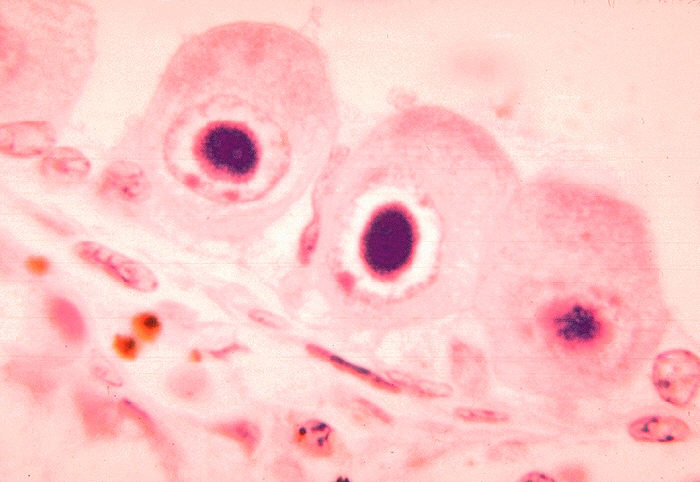
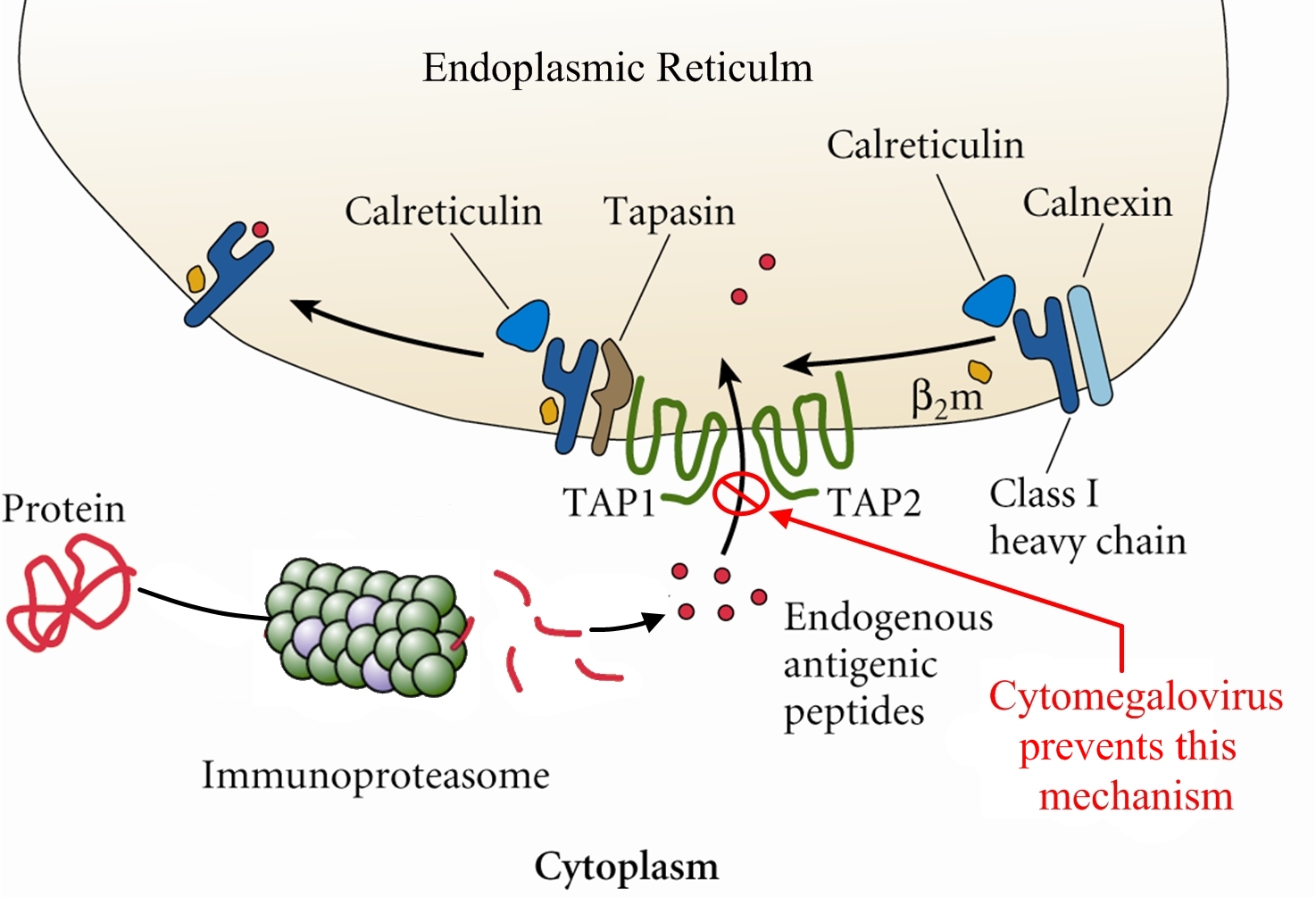
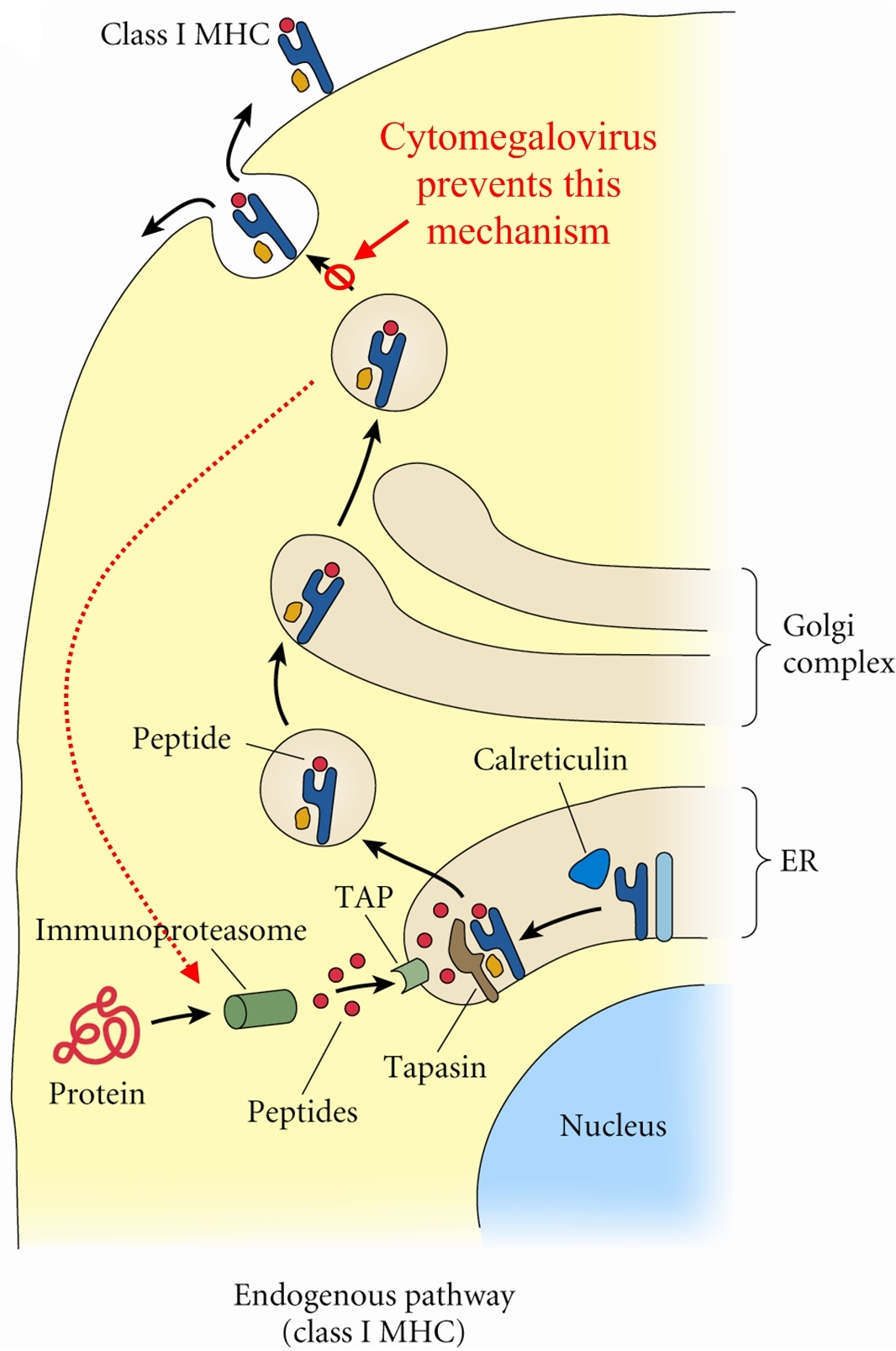
Overview: Cytomegalovirus (CMV) is a double-stranded, enveloped DNA virus and a member of the Herpesvirus family of DNA viruses, which contains over 100 different members, eight of which infect humans (Figure 1). The host-cell-derived envelop of CMV encloses an icosahedral (20-faced) nucleocapsid structure that is roughly 150 nm (nanometres) in diameter. Infections caused by CMV often produce perinuclear cytoplasmic and nuclear inclusions, and clinical infections associated with this pathogen vary depending on the host's age, antibody status, and overall immune response. Isolated strains of CMV demonstrate considerable genomic and phenotypic heterogeneity, except for epidemiologically related partners, such as mothers and their children. Figure 1. Histopathology of cytomegalovirus infection in the kidney. Pathogenicity: CMV infects both epithelial cells and various cells of the immune system. Generally, once a virus is engulfed by an antigen-presenting cell, such as a macrophage or B lymphocyte, its genomic material is transcribed into proteins via the host's protein-producing machinery. Typically, viral proteins that are produced via this route, called the endogenous route, are immediately digested by immunoproteasomes found in the cytoplasm and subsequently presented on the surface of the infected cell using MHC class I molecules, so that an immune response can immediately be generated. However, CMV exploits this mechanism by preventing viral peptides from being presented on the surface of infected cells. It does this by blocking digested protein remnants from moving into the endoplasmic reticulum via transmembrane peptide transporters known as TAP-1 and TAP-2. Both transporters use ATP energy to bind semi-digested protein antigens to MHC class I molecule, where they are presented on the surface. CMV evades this mechanism by binding to TAP and blocking its transport ability, resulting in viral proteins remaining in the host cytoplasm, thus resulting in an accumulation of viral antigens within the infected cell and no subsequent immune response (Figure 2). Figure 2. Endogenous pathway – proteins are broken down into peptides in immunoproteasomes and transported by TAP into the endoplasmic reticulum. Click to enlarge. CMV has also been shown to bind to MHC class I molecules and prevent them from leaving the endoplasmic reticulum or Golgi apparatus - the secondary destination of MHC class I molecules prior to being presented onto the surface (cell membrane) of the antigen-presenting cell. Similarly, CMV can induce bound-MHC class I molecules to be translocated into the cytosol where they are subsequently degraded by host cell immunoproteasomes (Figure 3). This form of MHC class I molecule suppression is caused by the viral effector molecule US11. Figure 3. Assembly & loading of class I MHC with exogenous peptides and transport to cell surface. Click to enlarge. Clinical Infections: Congenital CMV is the most serious disease caused by this virus and about 1% of infants born worldwide excrete CMV from their urine at delivery as a result of infection in utero. Upon physical examination, 90% of these infants appear normal, but go on to develop sensory nerve hearing loss/psychomotor mental retardation later in life. Infected infants at birth show several defects and disorders such as jaundice, anemia, thrombocytopenia, low birth weight, microcephaly, and chorioretinitis. Neonatal infection has no adverse reaction and most infections during childhood and adulthood are completely asymptomatic. In immunocompromised patients, such as those with AIDS or cancer, latent CMV can be reactivated resulting in diffuse involvement of lung and severe hypoxia, leading to a condition known as CMV pneumonia. Interstitial pneumonia caused by CMV is the leading cause of death in patients receiving bone marrow transplant. The virus can also disseminate to visceral organs in AIDS patients causing hepatitis (inflammation of the liver). At least 80% of adults have antibodies to the CMV virus and is most prevalent in children up to the age of five. The rate increases in young adults, most likely as a result of sexual transmission of the disease. The virus has been isolated in bodily fluids such as saliva, semen, blood, tears, breast milk, cervical secretions, urine and white blood cells. Latent infection which reside primarily in leukocytes and their precursors cause the disease type associated with blood transfusions. Immunology: CMV is diagnosed by showing a rise in antibody titer by isolating the virus or finding viral particles in the fluid by electron microscopy. The virus can be readily grown in serially propagated diploid fibroblast cell lines. The presence of
References: Sherris, C.J. (1990). Medical Microbiology: An introduction to infectious diseases. Elsevier Science Publishing Co., New York. |