|
Epstein-Barr Virus
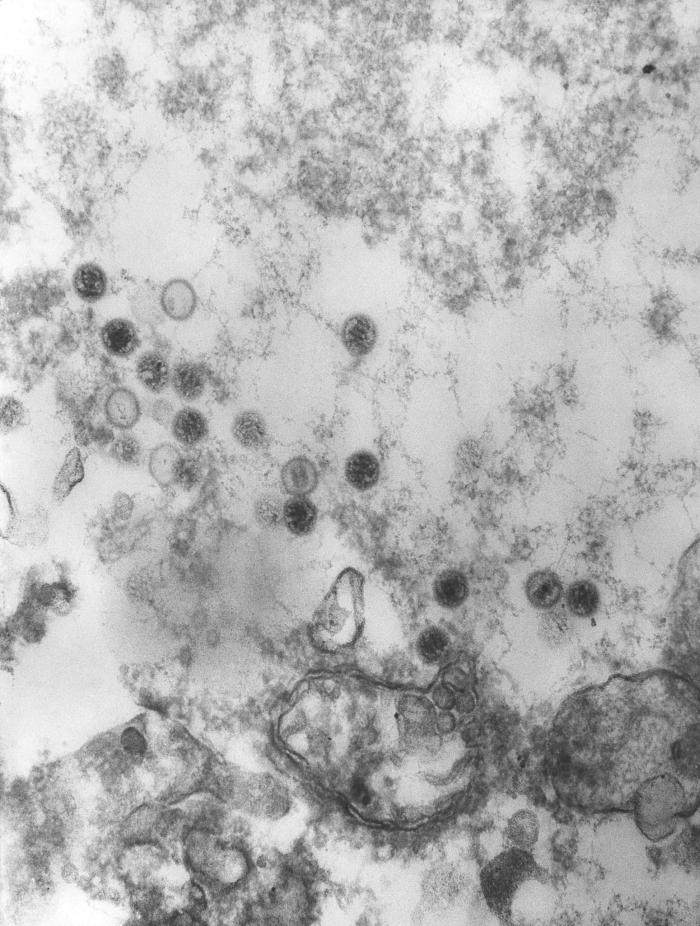
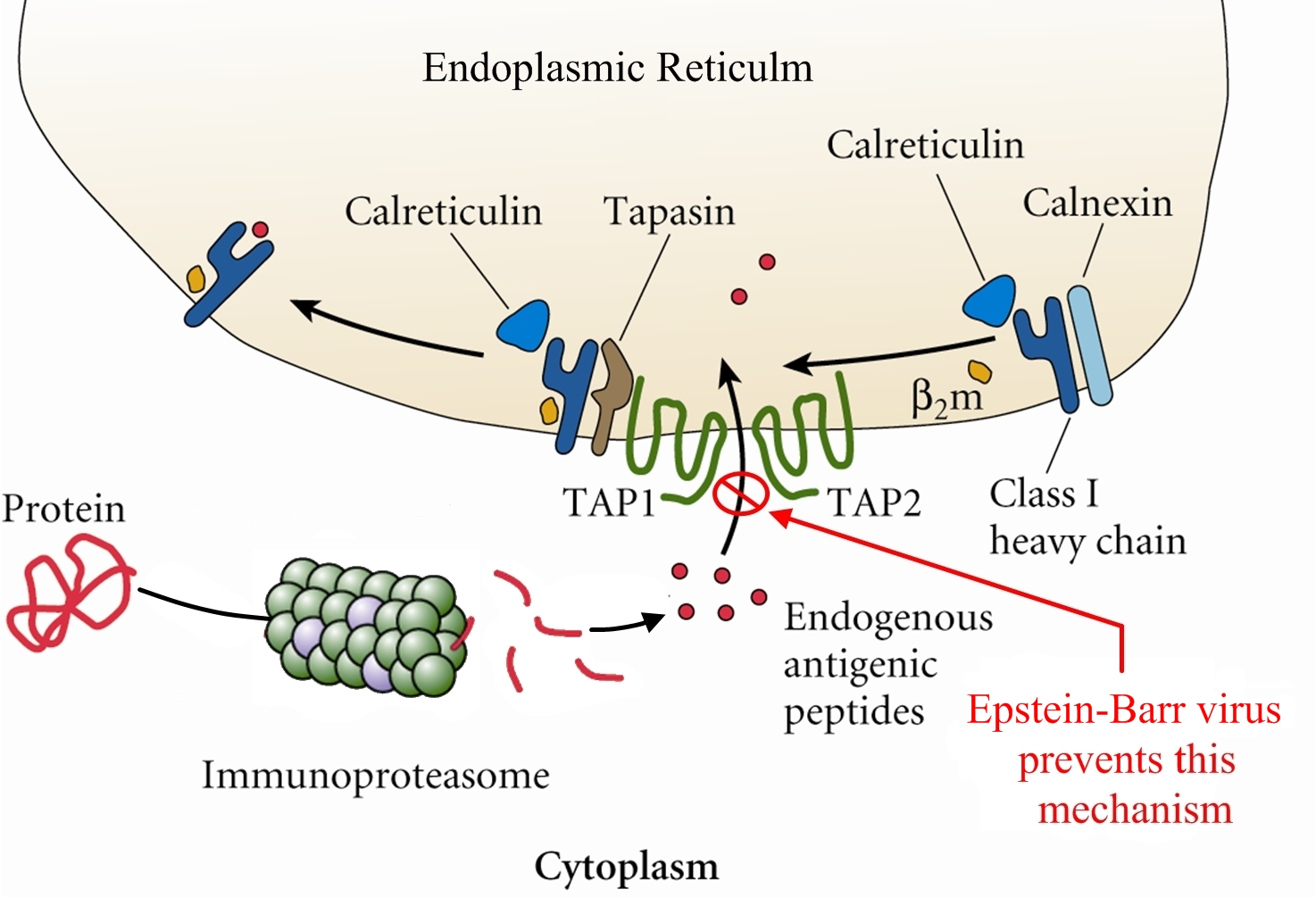
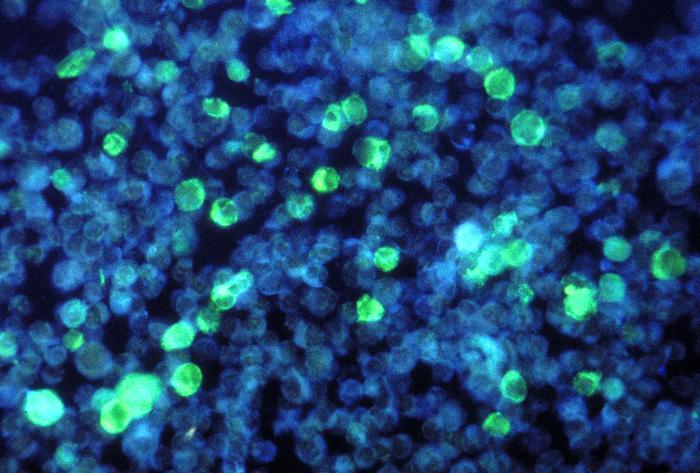
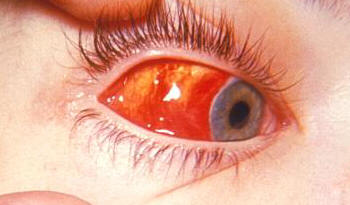
Overview: The Epstein-Barr virus (EBV) is an enveloped virus with an icosahedral-shaped (20-faced) capsid that is 100 nm (nanometres) to 110 nm in diameter, containing double-stranded linear DNA of approximately 175 kilobase pairs (Figure 1). The virus is a member of the Herpesvirus family of viruses, and is responsible for infectious mononucleosis, Burkitt’s lymphoma, and nasopharyngeal cancer. The outside surface of the host-cell-derived envelope contains membrane-bound glycoproteins that aid in docking the virus and binding to receptors on targeted host cell. There is a space between the envelope and the capsid called the tegument. Within the tegument, there are clusters of proteins that aid in viral DNA replication. Figure 1. This negatively-stained transmission electron micrograph reveals the presence of numerous Epstein-Barr virus virions. Pathogenicity: EBV infects B lymphocytes that express the receptor for complement C3d proteins, known as CD21. EBV begins its infection by attaching to a B lymphocyte with glycoproteins 350 and 220 found on its external envelope. Cross-linking of the surface proteins on the B cell induces entry into the target cell through endocytosis. EBV utilizes the B cell's machinery to replicate its DNA and produce viral proteins within the epithelial cells of the upper respiratory tract. Typically, viral proteins that are produced via this route, called the endogenous route, are immediately digested by immunoproteasomes found in the cytoplasm and subsequently presented on the surface of the infected cell using MHC class I molecules, so that an immune response can immediately be generated. However, EBV exploits this mechanism by preventing viral peptides from being presented on the surface of infected cells. It does this by blocking digested protein remnants from moving into the endoplasmic reticulum via transmembrane peptide transporters known as TAP-1 and TAP-2. Both transporters use ATP energy to bind semi-digested protein antigens to MHC class I molecule, where they are presented on the surface. CMV evades this mechanism, thereby causing viral peptides to remain in host cytosol (Figure 2). The virus is then shed into the infected host's saliva, making the virus available to be taken up by B lymphocytes expressing the co-receptor CD21. The activation of CD21 amplifies B cell receptor signaling and enhances the efficiency of the signaling process that leads to cell division. The infection causes an inhibition of the T cell responses, since T helper cells require antigen-presenting cells, such as B cells, for activation. As a result, B cells are protected from apoptosis (programmed cell death) and become mediators of persistent infections. A lack of apoptosis often causes secondary lymphoid organs such as the spleen to enlarge, especially since there is an increased number of both phagocytic cells and other lymphocytes. Figure 2. Endogenous pathway – proteins are brokendown into peptides in immunoproteasomes and transported by TAP into the endoplasmic reticulum. Click to enlarge. The virus also causes infected cells to produce interleukin-5 (IL-5) and interleukin-6 (IL-6) cytokines via T helper 2 cells, which stimulate the production of eosinophils and proinflammatory immune responses, respectively. Its genome also encodes an interleukin-10 (IL-10) -like protein (BCRF1) that binds to IL-10 host receptors and down-regulates the immune system by shutting off interferon-γ (INF-γ) production. INF-γ is the main chemical signal produced by cells of the immune system that induces an anti-viral effect by activating macrophages. Similarly, Epstein-Barr virus' genome produces yet another effector molecule, this time a homologue of the bcl-2 gene product called BHRF1, that induces apoptosis. In the disease mononucleosis (usually referred to as mono for short), EBV is transmitted from person to person by the transfer of saliva; consequently, the disease is sometimes referred to as the 'kissing disease'. Common symptoms of this disease include pale complexion, a tired feeling, and a lack of energy sometimes to the point of not being able to get out of bed. Interestingly, when infection with EBV occurs during adolescence, or young adulthood, it causes infectious mononucleosis 35% to 50% of the time. Typically, a period between one and four weeks is required to clear the infection; however, the infected host will continue to shed the virus for many years and possibly remain dormant in the person's immune system for the rest of his/her life. Identification: Large quantities of immunoglobulin M (IgM) antibodies bound to viral capsid antigens in the blood is an indication that a person may be infected with EBV. Laboratory diagnosis of the EBV is made by testing blood serum samples for the antibodies to several EBV-associated antigens using standard immunofluorescence assays (Figure 3). Confirmation of the virus involves antibody titrations of IgM and immunoglobulin G (IgG) to EBV viral capsid antigen, as well as IgM to the early antigen and the nuclear antigen. Figure 3. This photomicrograph depicts leukemia cells that contain Epstein-Barr virus using a fluorescent antibody staining technique. Clinical Infections: EBV causes chronic infections throughout a persons lifetime and is continually secreted into the oral cavity via the salivary glands, making it highly contagious. The EBV can be oncogenic and was one of the very first viruses to be discovered as a causative agent of cancer (Rowe et al., 2009). The virus is considered oncogenic when its presence causes the activation of the Myc oncogene, leading to cell growth, proliferation, and a decrease in apoptosis (Bornkamm, 2009). At times non-infectious conjunctivitis, as well as other corneal abnormalities may manifest themselves due to the body’s systemic response to EBV infections, such as mononucleosis (Figure 4). Figure 4. A conjunctival hemorrhage of the eye with infectious mononucleosis. Furthermore, EBV is also the causative agent of Burkitt’s lymphoma, a type of cancer that effects the lymphatic system, in particular, B lymphocytes. The disease is often associated with the growth of tumours in the jaw and face and it is more prevalent in children. The disease is currently an endemic in Africa. In order to treat this condition, dose-adjusted chemotherapy drugs with rituximab are required. Effect of the chemotherapy, as with all cancers, depends on the time of diagnosis. Similarly, nasopharyngeal cancer is a disease in which malignant cancer cells form in the tissues of the nasopharynx - more specifically, epithelial cells of the upper respiratory tract. Uncontrolled growth of these cells is often due to the insertion of EBV DNA into the DNA sequence of infected cells. Unlike Burkitt’s lymphoma, however, this disease is more prevalent in China and east Africa. Nasopharyngeal cancer can be treated with surgery, chemotherapy, or radiotherapy. References: Bornkamm, G. (2009). Epstein-Barr virus and its role in the pathogenesis of Burkitt’s lymphoma: an unresolved issue. Seminars in Cancer Biology, 19: 351-365. Rowe, M., Kelly, G.L., Bell, A.I., & Rickinson, A.B. (2009). Burkitt’s lymphoma: the rosetta stone deciphering Epstein-Barr virus biology. Seminars in Cancer Biology, 19: 377-388. |