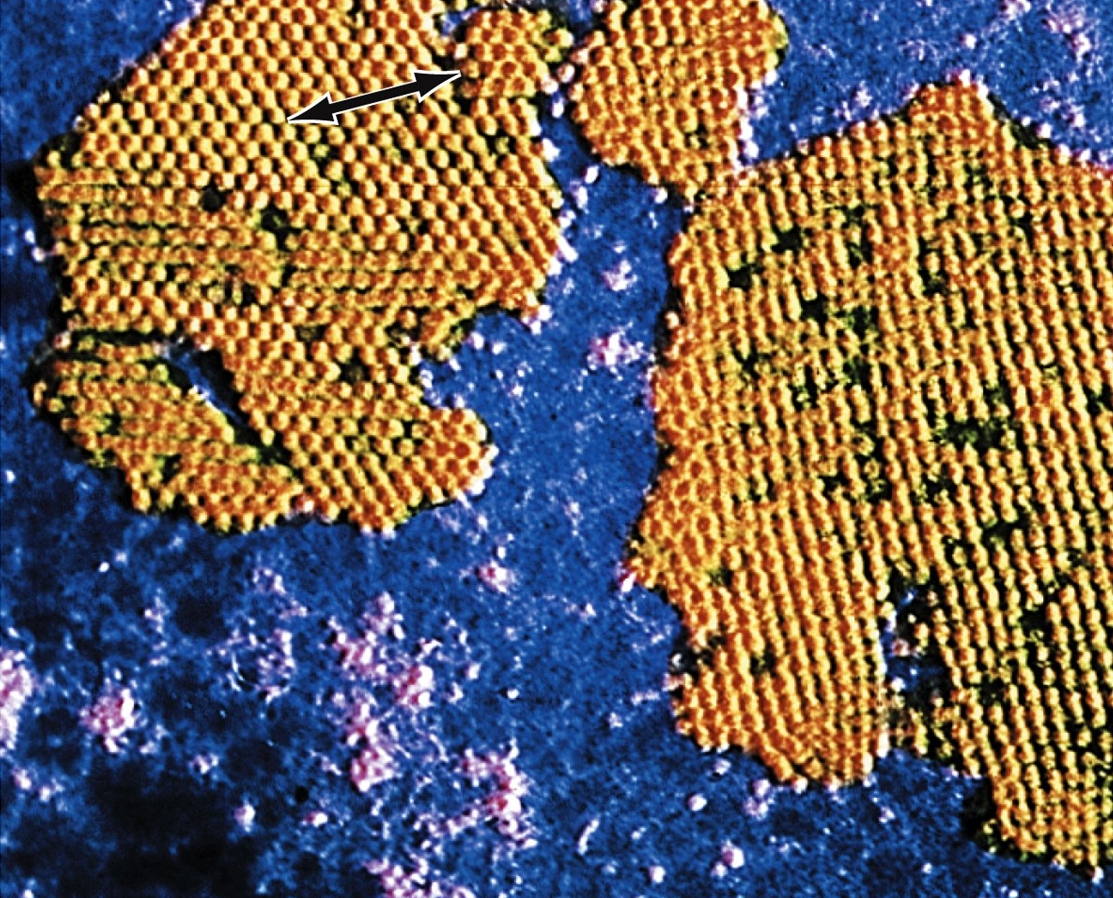
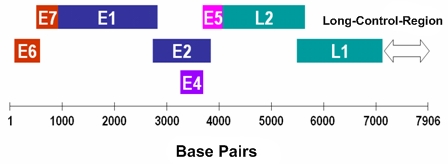
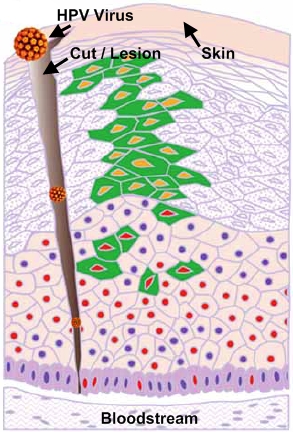
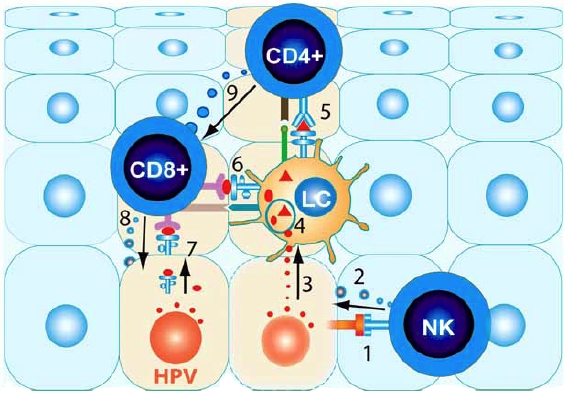
Human PapillomavirusOverview: Human papillomavirus (HPV) is a non-enveloped, double-stranded circular DNA virus of approximately 8000 base pairs and roughly 55 nm (nanometres) in diameter (Figure 1). The virus is a member of the Papovavirus family, which consists of over 100 genotypes, causing many different infections in people of all ages. Most infections are harmless, but about 30 types may lead to cancer. Genital HPV is the most common sexually transmitted infection, with more than 40 known types that can infect both males and females in their genital areas, anus, mouth and throat, by causing genital warts or cancers. The epithelial cells of the skin and mucous membrane are primarily infected, but infections vary based on the quantity of virus particles, the type, intensity, length of contact, and the immunity status of the patient (Tchernev, 2009). Figure 1. Micrograph depicting multiple Human papillomavirus virions. Pathogenicity: The virus preferentially targets squamous epithelial cells. Its genomic structure contains a long control region which has up to eight open reading frames that encode for non-structural and capsid proteins (Figure 2). E1, E2 and E4 proteins are indispensable for viral replication, regulation of transcription and genome amplification, respectively. E5, E6 and E7 proteins have transforming functions such as activation of the epidermal growth factor receptor and the phosphatidylinositol 3-kinase (PI3) signaling pathway, promotion of p53 degradation, and inactivation the retinoblastoma protein, respectively. Figure 2. Linear representation of HPV16 genome. The life cycle of HPV is tightly linked to differentiation of the cervical epithelium beginning with the infection of basal cells most likely through negligible lesions (Figure 3). In the infected basal cells, the viral genome is transcribed into a bicistronic message that encodes the early E6 and E7 proteins, which promote cellular proliferation. As these cells migrate toward supper epithelial layers and differentiate, expression of E6 and E7 is switched off and new transcripts are released encoding proteins required for viral genome amplification, most notably E4. Finally, a number of the E4-expressing cells synthesize the capsid proteins L1 and L2, required for assembly of infectious viral particles. In some cells, however, productive infection is aborted and the viral DNA may integrate into the host genome from where the E6 and E7 genes are constitutively transcribed. HPV can remain latent for long periods, get eliminated by the immune system, or become activated and initiate infection. Continuous expression of E6 and E7 proteins causes cellular transformation leading to increasingly severe epithelial lesions known as cervical intraepithelial neoplasia (CIN), which may eventually progress to invasive cervical cancer. Figure 3. Human papillomavirus initially infects basal keratinocytes. Clinical Infections: Most HPV infections go unnoticed because they do not cause any symptoms. The virus may have been contracted years ago and it can remain in the body for weeks, years, or even a lifetime without showing any symptoms of an infection. However, for those who experience symptoms, the type of symptoms depends on the type of HPV infection. Common warts are painless, firm growths with a rough surface and appear on the knees, face, fingers, and around the nails. Flat warts are small, smooth warts appearing in clusters on the back of the hands, face, or legs. Plantar warts are those appearing on the soles of the feet. They can be painful because of their weight-bearing location on the feet. Filiform warts form long, thin projections around the eyes, face, and neck. Genital warts are small, cauliflower-shaped, or flat lesions. They occur on the genital areas including the vagina, cervix, vulva, penis, scrotum, and anus. They are usually painless but they can bleed, itch, or have some discharge. Precancerous lesions or cervical dysplasia are abnormal cells in the cervix. These are painless and can only be detected with a Pap smear. Individuals that are sexually active with many partners increase their chances of being infected; however, it can also spread by contact, such as mouth to mouth, or passed on to infants during pregnancy with an infected mother. Individuals that are immunocompromised are at a greater risk and more likely to experience an aggressive course of infection because of the importance the immune system has in regulating HPV infections. Treating and Controlling HPV: Some minor CIN lesions regress as a result of effective innate and adaptive cytotoxic responses (Cid-Arregui, 2009). Figure 4 depicts a natural killer cells getting activated by NKG2D receptor ligands expressed on the infected cell’s surface, and releasing cytolytic granule contents. Langerhans cells take up the viral antigen and present viral epitopes to CD4+ and CD8+ T cells, which become activated. Activated CD8+ T cells will later release granules that contain granzymes, which cause cell death in target cells. These CD8+ T cells are stimulated by cytokines that are released by CD4+ T cells upon their activation (Cid-Arregui, 2009). Figure 4. Summary of the conditions that allow recognition and regression of HPV infected cells by effective immune cells. Vaccination and Treatment: Unfortunately, there is no cure for HPV itself. There are preventative vaccines, such as Gardasil and Cervarix, which block initial HPV entry into epithelial cells (Lin et al., 2010). For the existing HPV infection, vaccines have been generated to decrease the infection and neoplasms associated with T cell-based immune responses (Lin et al., 2010). There are also treatments available for the associated health problems, such as genital warts, cervical changes, and cervical cancer; however, the virus remains in the body. With natural HPV infections there is a type-specific response from the detectable neutralizing antibodies; on the other hand, vaccines create type-specific and cross-neutralizing antibodies, since both Gardasil and Cervarix have shown evidence of protection against non-vaccine HPV types. For HPV kinds that lead to cancer, there are polymerase chain reaction (PCR) and DNA hybridization tests available to detect the virus even before there are major anatomical changes to the host (Tchernev, 2009). References: Cid-Arregui, A. (2009). Therapeutic vaccines against human papillomavirus and cervical cancer. The Open Virology Journal, 3: 67-83. Lin, K., Doolan, K., Hung, C., & Wu, T.C. (2010) Perspectives for Preventive and Therapeutic HPV Vaccines. Formos Medical Association, 109(1): 4-24. Letian, T., & Tianyu, Z. (2010). Cellular receptor binding and entry of human papillomavirus. Virology Journal, 7: 1-7. Tchernev, G. (2009). Sexually transmitted papillomavirus infections: epidemiology pathogenesis, clinic, morphology, important differential diagnostic aspects, current diagnostic and treatment options. Anais Brasileiros de Dermatologia, 84(4): 377-389. |