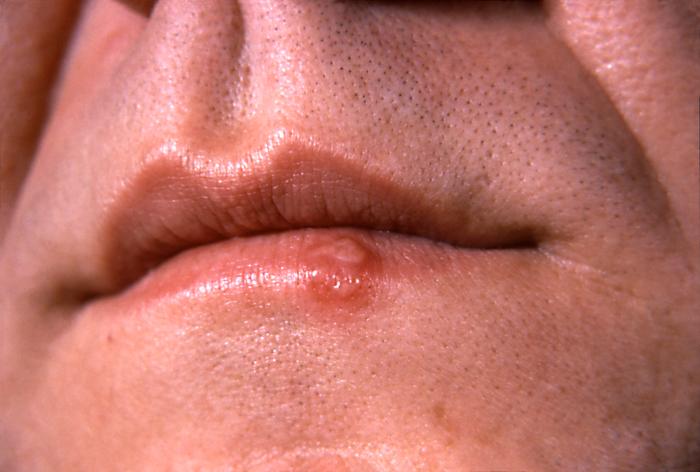
Herpes Simplex VirusOverview: Herpes simplex virus (HSV) is an enveloped, doubled-stranded DNA virus and a member of the Herpesviridae family (Figure 1). The organism possesses an icosahedral capsid and is considered to be relatively large for a virus, with virions ranging from 120 nm (nanometres) to 300 nm in size. A virion consists of an electron-dense core containing the viral genome, an icosahedral capsid around the core, an amorphous tegument around the capsid, and an envelope derived from cellular membranes containing both glycosolated and nonglycosylated proteins, lipids, and polyamines. The variability in the size of herpes virions is due mainly to variability in the make-up of the tegument and the state of the envelope. There are two types of HSV, namely, types 1 (HSV-1) and type 2 (HSV-2). HSV-1 is known for causing cold sores (Figure 2), while HSV-2 is most commonly associated with genital herpes. Although this is generally the case, both types are capable of causing either infection. HSV is also known for causing keratoconjunctivitis, which is the inflammation of the cornea and conjunctiva. Figure 1. Computer-animated depiction of herpes simplex virus morphology. Figure 2. This is a close-up of a herpes simplex lesion of the lower lip on the 2nd day after onset. Also known as a cold sore, this lesion is caused by the contagious herpes simplex virus Type-1 (HSV-1), and should not be confused with a canker sore, which is not contagious. Pathogenicity: Generally, the virus infects oral or genital mucosal epithelial cells. The initial association is between proteoglycans of the cell surface and glycoprotein C (gC) (Figure 3). This is followed by a specific interaction with one of several cellular receptors collectively termed 'HVEM' for herpesvirus entry mediators. These are related to receptors for nerve growth factors and tumor necrosis factor. The association requires the specific interaction with the glycoprotein gD. Fusion with the cellular membrane follows (Figure 3). This requires the action of a number of viral glycoproteins including gB, gH, gI, and gL. The viral capsid with some tegument proteins, namely, VP1-3 protein - an extremely large 336 kDa (kiloDalton) protein, thought to be involved in nucleocapsid attachment to the nuclear pore facilitating DNA release into the nucleoplasm, then migrate to nuclear pores along cellular microtubules utilizing cellular transport machinery (Figure 3). This docking is thought to result in the viral DNA being injected through the pore while the capsid remains in the cytoplasm. Another tegument protein, such as alpha trans-inducing factor (a-TIF), enters the nucleus with the viral genome. The following mechanism is summarized in Figure 3. Figure 3. Computer-animated depiction of viral entry into a target cell. Viral Latency: The HSV has latency within the body; the reoccurrence of the infection is called the recurrent infection. The recurrent infections can be initiated by a number of factors including stress, fever, and exposure to ultraviolet light (Whitley et al., 1998). Latent infection and reactivation by HSV takes place in sensory neurons, primarily in the trigeminal ganglia for HSV-1. The process of establishment involves virus entering neurons at the periphery, and the viral genome traveling up the axon and entering the nucleus. While some neurons are destroyed as virus replicates, most neurons are refractory to virus replication and the viral genomes become associated with host histones and persist as mini-chromosomes (Figure 4). Figure 4. Computer-animated depiction of latent infections caused by herpes simplex virus. The expression of most viral
genes is absent during latent infection, but a
number of latently infected neurons express a
single transcript - the latency associated
transcript or LAT - which is encoded in the
repeat regions of the genome (Figure
4). LAT expression facilitates reactivation,
but its mechanism of action is unclear at this
time. It does not appear to directly involve the
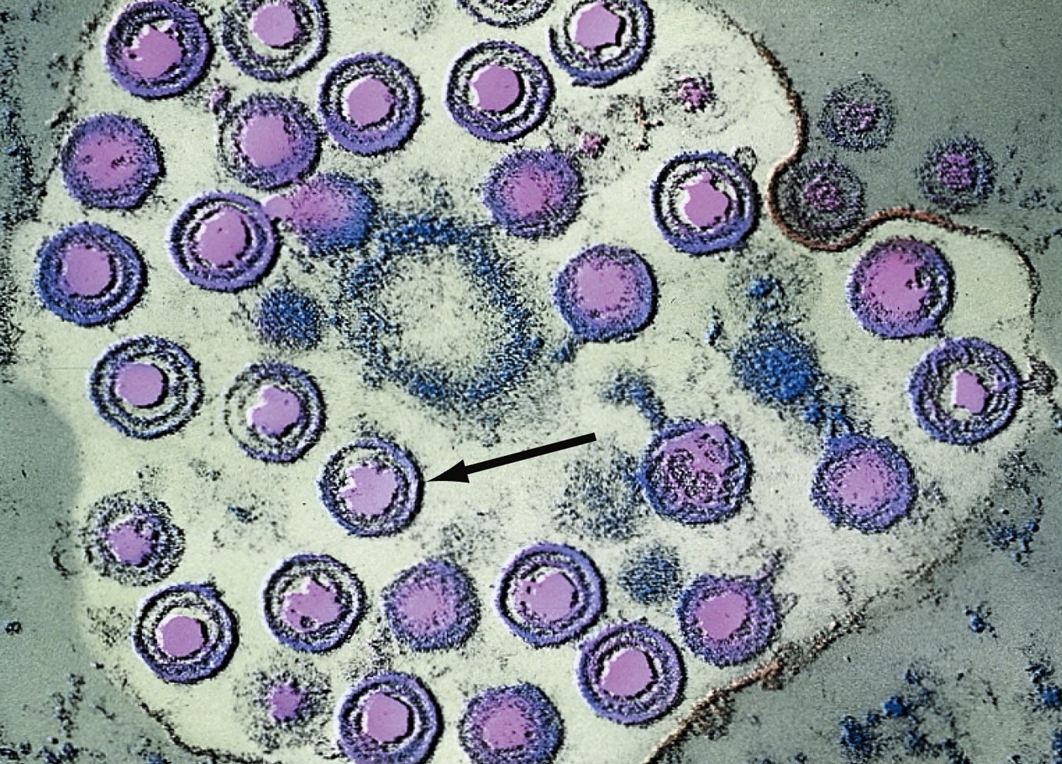
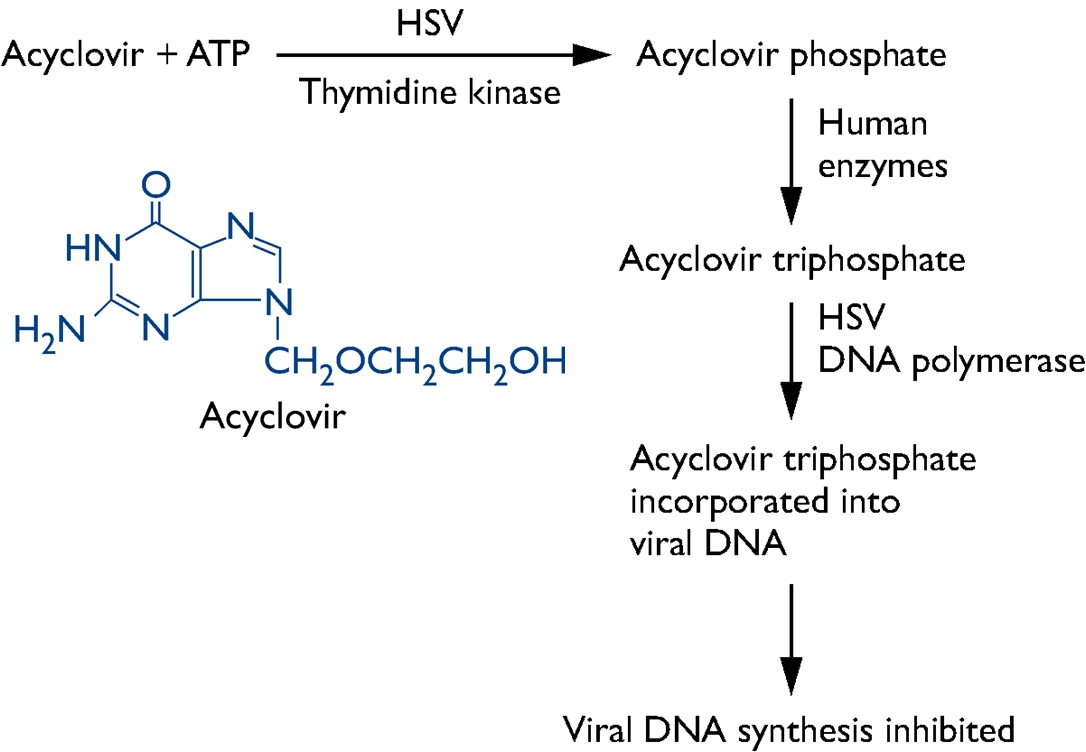
expression of a protein. Moreover, the recurrent infections can occur despite the efforts of both innate and adaptive immunity (Whitley et al., 1998). HSV is capable of hindering the immune system by inhibiting an endoplasmic reticulum-peptide transporter known as TAP. As a result of this, the antigen is not presented and the MHC class I molecules build up in the endoplasmic reticulum (Pier et al., 2004). Identification and Transmission: An infection with HSV is usually diagnosed by isolating the virus by scraping the skin lesions and a blood test can also be used to look for antibodies for HSV, indicating there has been an infection (Figure 5). Visually examining the sores also can indicate an infection (Figure 2). Furthermore, more than one third of the world’s population has been infected with HSV (Whitley et al., 1998). The rate of infection for HSV-1 is affected by location, socioeconomic status, and age. For example, 70 to 80% of those with low socioeconomic status have been infected by HSV-1 by adolescence (Whitley et al., 1998). HSV-2 infection typically do not occur before the age of sexual activity and risk is associated with related behaviours. Being immunocompromised increases the risk for more severe infections. For instance, herpes may play a role in the spread of HIV, the virus that causes AIDS. Herpes can make people more susceptible to HIV infection, and it can make HIV-infected individuals more infectious. Figure 5. This transmission electron micrograph reveals the presence of numerous herpes simplex virions. The arrow points to a single virion. Clinical Infections: Primary HSV-1 infection can be either totally asymptomatic or can result in a symptoms in any combination of fever, sore throat, vesicular or ulcerative lesions. However, asymptomatic infection is generally the rule rather than the exception. The duration of symptomatic disease in symptomatic children is generally two to three weeks, with a fever of 37°C to 38°C. The onset of a recurrent HSV-1 infection is generally marked pain, burning, tingling, or itching, which generally lasts for less than six hours, and is followed by vesicle formation within 24 to 48 hours. Usually, three to five vesicles appear at the border of the lip and last no longer than 48 hours. Pain is most severe at the outset of vesicle formation and resolves in four to five days. In HSV-2, the most severe clinical symptoms are encountered with primary infection, characterized by the appearance of macules and papules followed by vesicles, pustules, and ulcers. The duration of lesions and viral secretion averages about three weeks. Men and women experience both similar and dissimilar symptoms. Pre-existing immunity to HSV-1 can have a beneficial effect in reducing the severity of HSV-2 primary infections. Recurrent HSV-2 is milder than initial infection and is characterized by the appearance of three to five vesicles. Symptoms usually last seven to ten days, and virus is shed for an average of two to five days. The biggest problem involving recurrent genital herpes is the frequency of recurrences, which varies by individual. Recurrences usually occur several times per year; and, whether symptomatic or asymptomatic, transmission of the infection to sexual partners can occur with intimate contact. Moreover, genital HSV (HSV-2) can lead to potentially fatal infections in babies. It is important that women avoid contracting herpes during pregnancy because a newly acquired infection during late pregnancy poses a greater risk of transmission to the baby. If a woman has active genital herpes at delivery, a cesarean delivery is usually performed. Fortunately, infection of a baby from a woman with herpes infection is rare. Prevention and Treatment: The two methods for control of HSV infections are antiviral therapy and prevention. Anti-viral drugs such as acyclovir and valaciclovir are effective in limiting the extent of HSV infection and therefore helpful in limiting spread to uninfected individuals. In particular, acyclovir is a guanosine analogue that disrupts HSV DNA synthesis (Figure 6). However, post-exposure antiviral treatment does not prevent lifelong infection of an individual. Prevention of HSV infection is mainly achieved through avoiding contact with infectious secretions. Vaccination would be the ideal method of HSV prevention; however, to date no HSV vaccine has been clinically successful.
Figure 6. Mechanism of action of acyclovir and its chemical structure. References: Pier, G., Lyczak, J., & Wetzler, L. (2004). Immunology, Infection, and Immunity. American Society for Microbiology. Washington, DC. Whitley, R.J., Kimberlin, D.W., & Roizman, B. (1998). State-Of-The-Art Clinical Article. Clinical Infectious Diseases, 26: 541-553. |
|