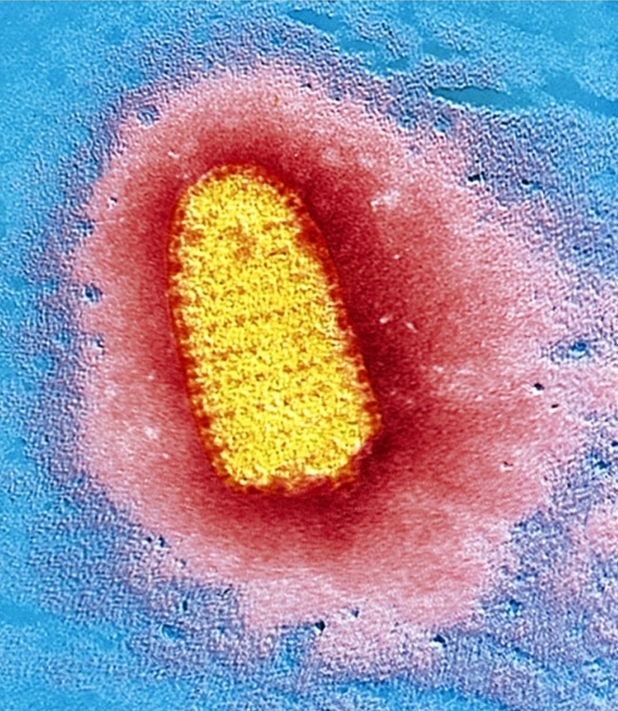
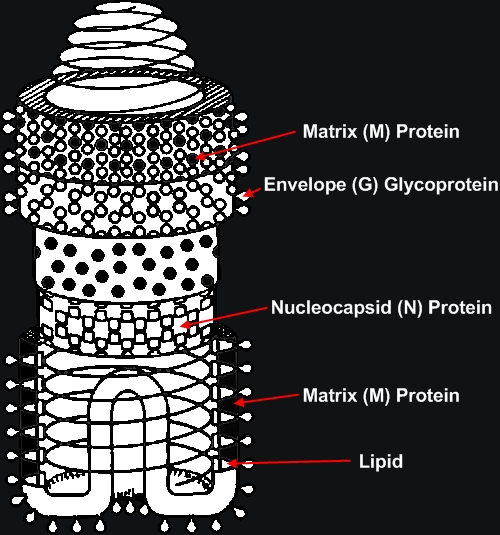
Rabies VirusOverview: Rabies virus is a bullet-shaped, single-stranded negative-sense, enveloped RNA virus (denoted (-)ssRNA) and a member of the genus Lyssavirus of the Rhabdoviridae family that causes rabies (Figure 1). The virus has an average diameter of 75 nm (nanometres) with lengths varying between 130 and 300 nm. Rabies virions contain four major polypeptides of different molecular size. One of them constitutes the protein moiety of the viral nucleocapsid, which is a single-stranded helical protein, whereas the remaining three are considered to be components of the viral envelope (Albertini et al., 1997) (Figure 2). It has been estimated that the envelope of a rabies virion contains 1800 molecules of these glycoprotein polypeptides. Figure 1. This transmission electron micrograph reveals the bullet-shaped morphology of the rabies virus. Figure 2. Nucleocapsid arrangement of the rabies virus inferred from electron microscopy studies. The coil represents the RNA genome.
Pathogenicity: When a human is exposed to rabies virus, the pathogen replicates at the site of inoculation. Aided by the G protein, the viral envelope attaches and fuses with the host cell membrane. Invagination of the plasma membrane with clathrin-coated pits allows cytoplasmic absorption via pinocytosis. The virions aggregate with the large endosomes, and after fusion with their membranes, they initiate the uncoating and release of the viral ribonucleoprotein core into the cytoplasm. Since the rabies virus has a linear negative-sense RNA genome, messenger RNAs are produced to permit virus replication using the host cell machinery. In particular, translation of the genome occurs on the free ribosomes in the cytoplasm, and some posttranslational processing occurs in the endoplasmic reticulum and Golgi apparatus. The switch from transcription to replication is triggered by the ratio of leader RNA to N protein and this subsequently causes replication of positive RNA strands of the viral genome. Translation of the rabies genome then occurs, sequentially producing the N, P, M, G, and L protein components. Full-length copies of the positive strands become templates for the new viral negative-stranded RNA. As the virion is assembled in the host cell, the newly synthesized RNA is complexed with the N, P, and L proteins to create the ribonucleoprotein core. The matrix protein initiates coiling. After assembly, the new complete virions bud off from the host cell, and replication is complete. Transmission: The virus spreads between animals through saliva secretions from an infected animal, usually as a result of a bite that breaks the skin. Once progeny virions are released from the initial site of inoculation, they migrate to the central nervous system via the bloodstream where they subsequently infects targeted cells. To date, the only cases of human-to-human transmission were a result of organ transplantations from undiagnosed patients (Takayama, 2008). Rabies is a zoonotic disease and the main hosts of the virus vary depending on the geographic location. In the vast majority of the developed world, carriers of the virus are mostly wild carnivorous animals like raccoons, foxes, and bats. However, in areas such as Africa and China, dogs remain the most significant carriers of the virus. According to the World Health Organization, 55000 cases of rabies are reported annually with 95% of these cases occurring in Africa and China.
Clinical Infections:
The incubation period of the virus is
approximately one to three months in 60% of
reported cases, but can last as little as 15
days to well over one year. The incubation
period of the virus allows it to migrate from
the point of entry (muscle tissue) to the
peripheral nervous tissue. From the peripheral
nervous system, the virus migrates to the
central nervous system by following the flow of
the axoplasm of neurons at an average rate of 8
to 20 mm per day (Takayama,
2008). The symptoms of rabies begin to appear
once the virus enters the central nervous
system. Within a period of twenty days after the
incubation period, the symptoms of the infection
progress from those that are nonspecific such as
fever and malaise to confusion, agitation,
hydrophobia and aerophobia. The patients finally
fall into comas and ultimately die from cardiac
arrest or respiratory failure (Fu, 1997). There
is currently no cure for rabies after the onset
of symptoms. It has been suggested that patients
succumb to the disease as a result of neuron
function alteration rather than tissue
destruction since many genes from recovered
tissues had decreased gene expression. Such
genes include nitric oxide synthase and
5-hydroxytryptamine receptors (Fu, 1997). The virus is not known to target specific demographics; all mammals are at risk of contracting the virus. However, most of the cases of rabies that are reported occur in children that are between the ages of 5 and 15; this can be due to lack of education on the virus or a child’s naiveté. Other people at risk are rural inhabitants or people who have elevated contact time with animals. References: Albertini, A.A.V., Schoehn, G., Weissenhorn, W. & Ruigrok, R.W.H. (2008). Structural aspects of rabies virus replication. Cellular and Molecular Life Sciences, 65: 282-294. Fu, Z. F. (1997). Rabies and rabies research: past, present and future. Vaccine, 15: 20-24. Takayawa, N. (2008). Rabies: a preventable but incurable disease. Journal of Infection and Chemotherapy, 14, 14: 8-14. |