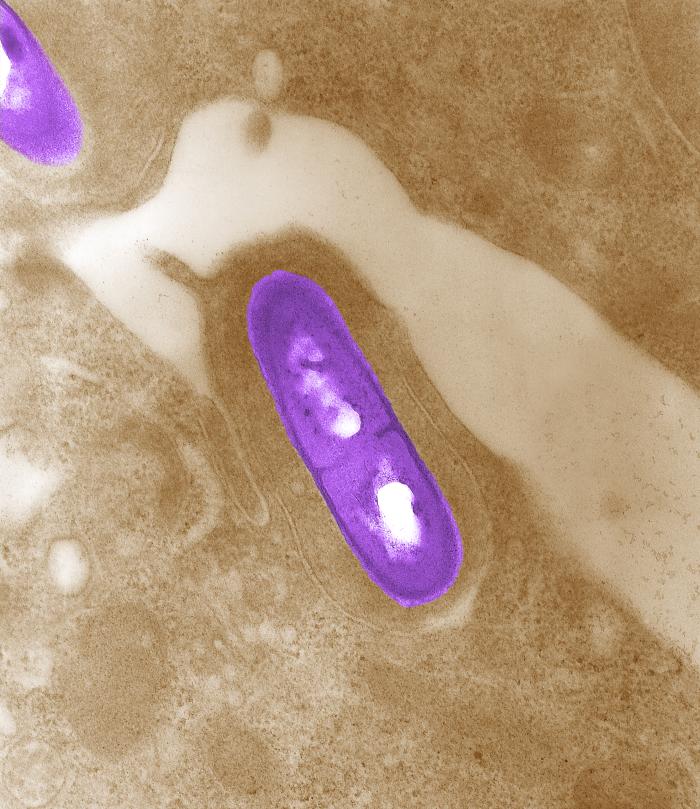
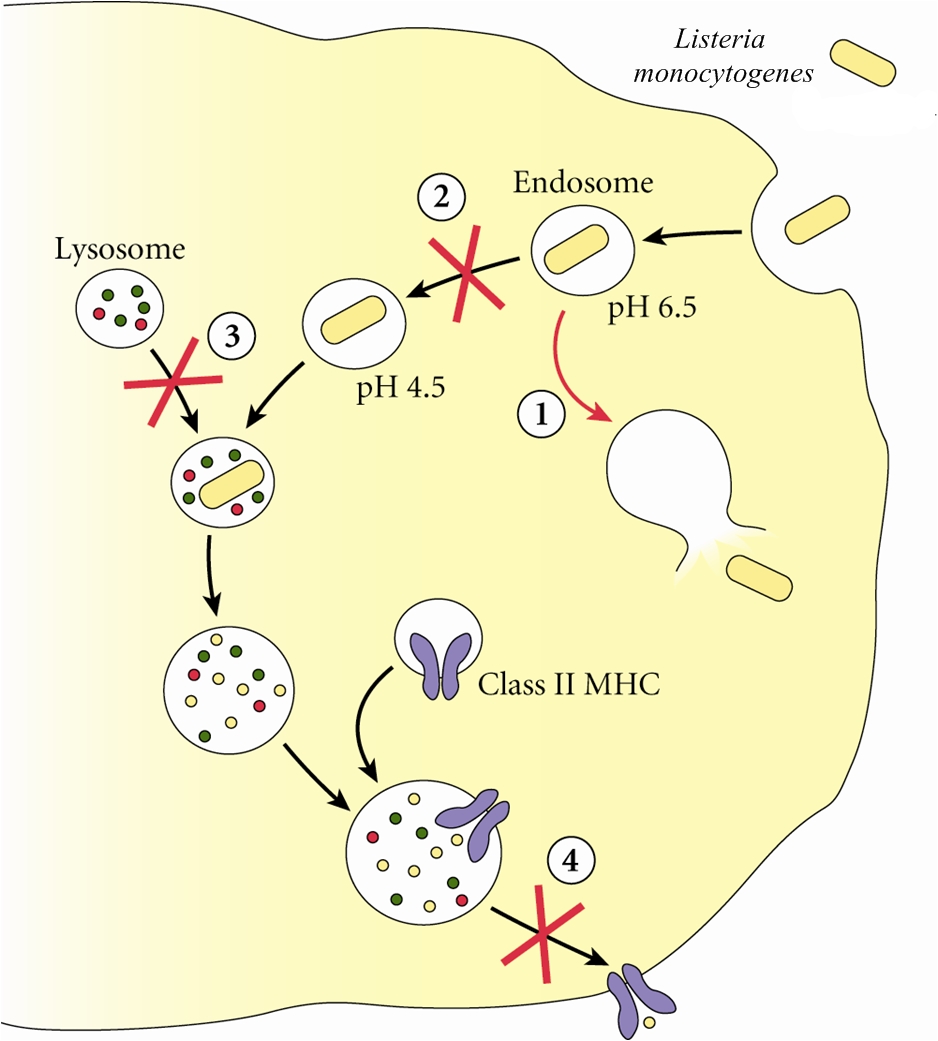
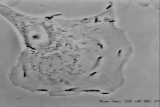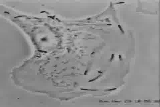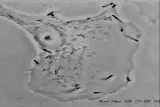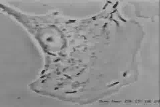
Listeria monocytogenesOverview: Listeria monocytogenes is a Gram-positive, motile, facultative intracellular food-borne bacterium that causes listeriosis in humans (Figure 1). This pathogen is widely distributed in nature due to its unique ability to withstand refrigeration temperatures and grow in environments of relatively high salt concentrations. Hence. L. monocytogenes is capable of growing in canned foods, especially those untreated with preservatives like sodium benzoate. Listeriosis is characterized by its ability to cause septicemia, meningitis, or spontaneous abortion in infected individuals, especially those who are immunocompromised (people with cancer or AIDS). Infection with this intracellular pathogen invokes a complex immune response characterized by the influx of neutrophils and a predominantly proinflammatory cytokine response. In the United States alone, 2500 people become ill from this bacteria and nearly 500 succumb to infection. Figure 1. Electron micrograph of a Listeria monocytogenes bacterium in tissue. L. monocytogenes bacteria are rod-shaped with round ends, measuring 0.4 to 0.5 μm (micrometres) in length by 0.5 to 2 μm in width, but may occur singly or in short chains (Holt et al., 1994). Smears that are 15 to 24 hours old tends to show cocci, and coccoid forms are seen in infected tissues. Moreover, flagella used for motility are produced at room temperature; temperatures exceeding 37°C prevent this biological mechanism from occuring (Holt et al., 1994). When oxygen supply becomes limited, this organism ferments glucose into lactic acid; however, acetate, ethanol, and carbon dioxide may also be produced. L. monocytogenes also expresses the exotoxin β-haemolysin, which produces zones of clearing on blood agar, indicating lysis of red blood cells. Pathogenicity: When L. monocytogenes is first introduced to its host, it is taken up by epithelial M cells of the intestine (the primary site of infection), where the bacteria invade non-phagocytic cells, and dentritic cells. The dentritic cells process the pathogen and present bacterial antigens on its surface via MHC class II molecules. Dentritic cells also secrete interleukin-23 (IL-23), which plays a critical role in regulating the production of IL-17A/F from nearby T-cells, thereby inducing the early presence of neutrophils in the liver during systemic infection with the cytoplasmic L. monocytogenes. A deficiency in either IL-23 or IL-17 receptor leads to increased mortality and decreased bacterial clearance from the spleen and liver. Thus, the regulation of IL-17A/F by IL-23 is critical in both the spleen and the liver (Meeks et al., 2009). IL-23 and IL-17 serves a different role in the spleen than in the liver that is independent of neutrophil recruitment and mobilization. Since IL-17A and IL-17F are known to induce the production of antimicrobial peptides in host cells, in addition to their ability to influence neutrophils, as in the liver, a lack of IL-17A/F in the spleen during L. monocytogenes infection reduces antimicrobial peptide production, thus affecting bacterial killing and clearance. It has been proposed that two separate immune pathways are activated during L. monocytogenes infection, namely, (1) IL-12 is secreted during early infection to induce the production of interferon-γ (IFN-γ) which activates macrophages to produce more effective phagolysosome and biochemicals, such as reactive oxygenated species and nitric oxide, and (2) Il-23 is secreted to induce the production of IL-17, influencing the mobilization and recruitment of neutrophils to sites of infection. Thus, a failure in either the IL-12 or IL-23 pathway can result in increased susceptibility to L. monocytogenes infection. Virulence: In order for L. monocytogenes bacteria to prevent an immune response from developing, they must escape MHC class II presentation on antigen-presenting cells, such as B-cells and dendritic cells. It does this by producing a haemolysin called listeriolysin O and two other enzymes: phospholipase A and phospholipase B. After a bacterium is taken up by a phagocyte, it is temporarly contained in a vacuole called an endosome or phagosome. The microenvironment of this vacuole has a pH of 5.9 to 6.5 (Figure 2). The toxin's cytolytic activity is maximized at a pH of 5.5, so listeriolysin O is selectively activated within the acidic phagosomes. The activation of this hemolysin and the secretion of phospholipase B causes the escape of the bacterium into the cytosol, where it can grow intracellularly. Upon release from the phagosome, the toxin has reduced activity in the more basic cytosol. Hence, listeriolysin O permits L. monocytogenes to escape from phagosomes into the cytosol without damaging the plasma membrane of the infected cell (Figure 2). This allows the bacteria to live intracellularly, where they are protected from extracellular immune system factors such as the complement system and antibodies. Figure 3. Immune evasion strategies used by Listeria monocytogenes to prevent being presented by MHC class II molecules. Click to enlarge. Furthermore, while the bacterium freely moves inside the cytoplasm, actin cytoskeleton protein (Arp2/3 complex) polymerizes around the pathogen in 'comet tail' formation; subsequently, L. monocytogenes is propelled unidirectionally into the host cell membrane and into adjacent cells (Figure 3). This does not occur if the species is unable to produce ActA protein - the protein that interacts with actin to increase actin polymerization. The protrusion which is formed may then be internalised by a neighbouring cell, forming a double-membrane vacuole from which the bacterium must once again escape using listeriolysin O and phospholipase B. Figure 3. L. monocytogenes propelling into an adjacent cell. Click to see an illustration. Treatment: When listeric meningitis occurs, the overall mortality may reach 70%. For septicemia, mortality may reach 50%, while for perinatal or neonatal infections, there is an 80% chance of succombing to infection. Mother's usually survive L. monocytogenes infections during pregnancy. Reports of successful treatment with parenteral penicillin or ampicillin exist. Trimethoprim-sulfamethoxazole has been shown effective in patients allergic to penicillin. References: Meeks, K.D., Sieve, A.N., Kolls, J.K., Ghilardi, N., & Berg, R.E. (2009). IL-23 Is Required for Protection against Systemic Infection with Listeria monocytogenes. Journal of Immunology, 183: 8026-8034. |