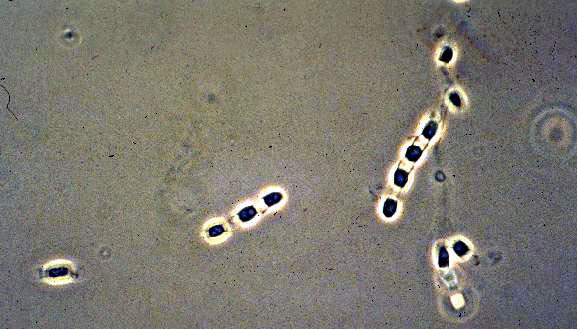
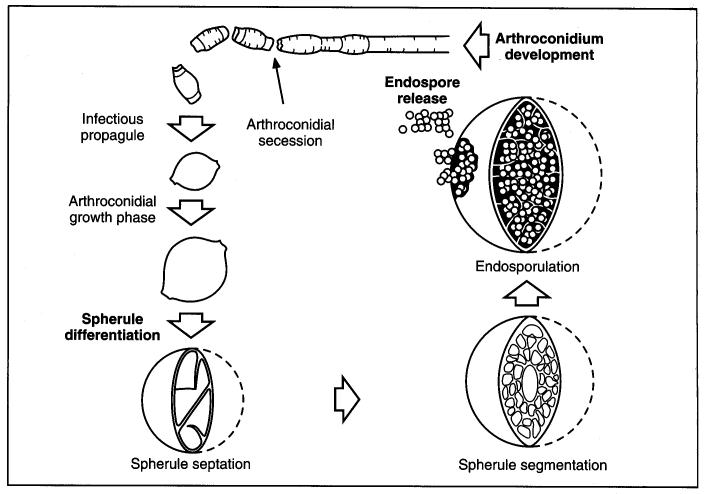
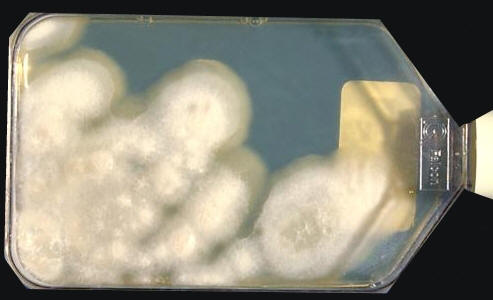
Coccidiodes immitisOverview: Coccidioides immitis is a dimorphic fungus that causes coccidioidomycosis or Valley Fever (Figure 1). This organism grows as a mould in the soil of warm and dry areas with low rain fall, and hence is endemic in certain parts of Arizona, California, Nevada, New Mexico, Texas, Utah and northwestern Mexico. The arthroconidia (arthrospores) produced by the hyphae are the infectious units of the organism. When they are inhaled, they produce spherules, thus propagating the infection. C. immitis colonies grow rapidly. The hematogenous spread of the pathogen in the host’s bloodstream results in infection of skin, bones, joints, lymph nodes, adrenal glands, and central nervous system. Figure 1. Arthroconidia of Coccidioides immitis. Life Cycle: The organism follows the saprophytic cycle in the soil and the parasitic cycle in vertebrates. The saprophytic cycle starts in the soil with arthrospores (mould form) that develop into mycelium. The mycelium then matures and forms alternating spores within itself. The arthrospores are then released, and germinate back into mycelia. The parasitic cycle involves the inhalation of the arthrospores by animals which then, at maturation, form spherules (30 μm (micrometres) to 60 μm in size), filled with uninucleated endospores (2 μm to 5 μm) (Figure 2). C. immitis is unique because it produces spherules containing endospores in tissue, and hyphae at 25°C. In addition, since C. immitis is an anaerobic organism, spherules produce rapidly in presence of CO2. Increased temperature and nutrition are also important for the production of sporulating spherules. Figure 2. The parasitic development of the spherule of Coccidioides immitis from an arthroconidium. A uninucleate arthroconidium (top) begins to swell and undergo mitosis to produce additional nuclei. Once mitosis stops, initiation of spherule septation occurs. The spherule is segmented into peripheral compartments with a persistent central cavity. Uninucleate endospores occurring in packets enclosed by a thin membranous layer differentiate within the compartments. As the endospores enlarge and mature, the wall of the spherule ruptures to release the endospores. Pairs of closely appressed endospores that have not completely separated from each other may resemble the budding yeast cells of B. dermatitidis. Click to enlarge. C. immitis colonies grow rapidly. The macroscopic morphology may be very variable. At 25°C or 37°C and on Sabouraud dextrose agar, the colonies are moist, glabrous, membranous, and grayish initially, later producing white and cottony aerial mycelium (Figure 3). With age, colonies become tan to brown in color. On the other hand, microscopic appearance of the fungus depends on the temperature of isolation. Figure 3. This is a Sabouraud’s dextrose agar culture of Coccidioides immitis with chloramphenicol and cycloheximide after two weeks.
Virulence & Pathogenicity: There are three main mechanisms in which C. immitis fungi use to survive in the hostile host environment. The first is the production of a dominant spherule outer wall glycoprotein (SOWgp) that modulates the host immune response, resulting in compromised cell-mediated immunity to coccidioidal infection. The second is the depletion of SOWgp presentation on the surface of endospores, which prevents host recognition of the pathogen when the fungal cells are most vulnerable to phagocytic defenses. Third is the induction of elevated production of host arginase I and coccidioidal urease, which contribute to tissue damage at sites of infection. Arginase I competes with inducible nitric oxide synthase (iNOS) in macrophages for the common substrate, L-arginine, and thereby reduces nitric oxide (NO) production and increases the synthesis of host orinithine and urea. Host-derived L-ornithine may promote pathogen growth and proliferation by providing a pool of the monoamine, which could be taken up and used for synthesis of polyamines via metabolic pathways of the parasitic cells. In fact, high concentrations of Coccidioides- and host-derived urea at infection sites in the presence of urease produced and released by the pathogen, results in secretion of ammonia and contributes to alkalinization of the microenvironment. Ammonia and enzymatically active urease released from spherules during the parasitic cycle of Coccidioides exacerbate the severity of coccidioidal infection by contributing to a compromised immune response to infection and damage of host tissue at foci of infection. Clinical Infections:
Coccidioidomycosis can be diagnosed
by immunodiffusion and serological tests that
detects the presence of fungal antigen or host
antibody IgM produced against the fungus
(Stevens,
2002). This disease can easily be targeted to
any healthy individual or animal that is exposed
to dust or dirt containing this pathogenic
fungus. However, this infection causes
significant mortality among individuals that are
immunocompromised (people with AIDS, cancer,
leukemia), transplant recipients, and
diabetic patients
(Chandler, 1985). It has also been evident that
coccidioidomycosis is not contagious from person-to-person. An individual must inhale the spores
of the
fungus in order to contract the disease.
Currently there are no such methods for
permanently eradicating C. immitis from the
soil; however, by taking simple environmental
measures such as planting grass, the amount of airborne Symptomatic infection (40% of cases) usually presents as an influenza-like illness with fever, cough, headaches, rash, and myalgia (muscle pain). Some patients fail to recover and develop chronic pulmonary infection or widespread disseminated infection (affecting meninges, soft tissues, joints, and bone). Severe pulmonary disease may develop in HIV-infected persons. Moreover, coccidioidomycosis may be divided into the following types, namely, (1) primary pulmonary coccidioidomycosis; (2) disseminated coccidioidomycosis; and (3) primary cutaneous coccidioidomycosis. Patients with self-limited disease or relatively localized acute pulmonary infections usually do not require antifungal therapy. Antifungal therapy should be given to patients who have disseminated disease or are under risk of complications due to their underlying immunosuppression and other factors. Amphotericin B and azoles, such as fluconazole, itraconazole, and ketoconazole are used for treatment of coccidioidomycosis. Animal experiments suggest that caspofungin, sordarins, and nikkomycins are also promising in treatment of coccidioidomycosis. References: Chandler, F. W. (1985). Pathology of the mycoses in patients with the acquired immunodeficiency syndrome (AIDS). Current Topics in Medical Mycology, 1: 1-23. Stevens, D.A. (2002). Diagnosis of fungal infections: current status. Antimicrobial Chemotherapy, 49: 11-19. |