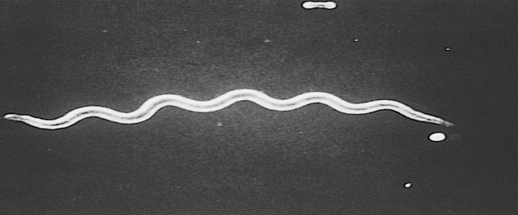
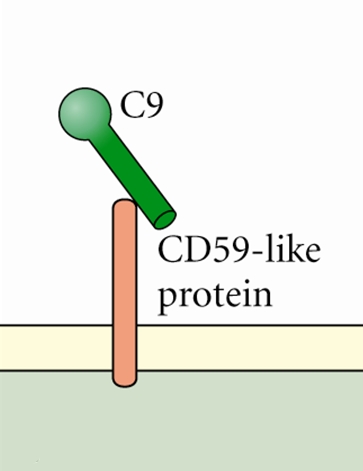
Borrelia burgdorferiOverview: Borrelia burgdorferi is a weakly-staining Gram-negative, tick-borne spirochetal bacterium and a member of the Spirochaetaceae family of the Spirochaetes class (Figure 1). This pathogen is typically 20 μm to 30 μm in length by 0.2 μm to 0.3 μm in width and possesses an axial filament composed of flagella, which run lengthways between its cell wall and outer membrane. This structure allows the spirochete to move efficiently in corkscrew fashion through viscous media, such as connective tissue. B. burgdorferi is optimally grown and isolated at 32°C in a microaerophilic environment and has a linear chromosome 1100 kilobases in size. Its genome encodes various unique outer surface proteins (Osp) that have been characterized A through F - the majority being OspA and OspB. These proteins are involved in the organism's virulence and transmission. In North America, Lyme disease (Lyme borreliosis) and Lyme neuroborreliosis (in the central nervous system), is caused by this zoonotic bacterium and is able to persistently infect a wide range of vertebrate species. Figure 1. Borrelia burgdorferi, a spirochete. Transmission: Ixodes scapularis, commonly known as the deer tick or blacklegged tick, is the main vector of Lyme disease in North America (Figure 2). They are known as the deer tick due to their habit of parasitizing the white-tailed deer. The Ixodes life cycle begins in the spring, particularly when eggs hatch to larvae. Upon hatching, the larvae parasitize either mice or deer and feed on their blood; if the animal is infected with B. burgdorferi, the larvae become a carrier of the bacteria as well and remain inside the parasite during its development. By the summer, individual larva grow larger in size, spreading the bacteria as they feed and grow, and subsequently encourage the growth of a larger reservoir for other parasites to become infected. The larva continues to develop during the fall and winter months, becoming a nymph by the following spring. The most frequent transmittance of disease occurs during this time, and continues until the following fall season, where the nymph matures to an adult tick. Development from egg to adult takes approximately two years, where adult ticks lay eggs and continue to feed until the animal perishes. When the tick is introduced to a human host and feeds on its blood, the pathogen disseminates into the salivary glands of the tick and enters the host. Two to three weeks after infection the bacteria spread throughout the body, establishing an infection. Figure 2. This photograph depicts a dorsal view of an adult female western blacklegged tick, Ixodes pacificus, which has been shown to transmit Borrelia burgdorferi, the agent of Lyme disease. The small scutum, or tough, chitinous dorsal abdominal plate, does not cover its entire abdomen, thereby allowing the abdomen to expand many times when this tick ingests its blood meal, and which identified this specimen as a female. The four pairs of jointed legs, places these ticks in the Phylum Arthropoda, and the Class Arachnida. Virulence and Pathogenicity: During early tick feeding, this pathogen remains within the gut, dividing within the blood nutrients being procured by the parasite. Down-regulation of the cellular surface protein OspA, a neutrophil stimulator, occurs during this time; consequently, becoming more OspA-negative. OspC upregulation is required during the initial stages of infection to avoid a host immune response. OspA-positive and OspC-negative bacteria are unable to infect a host as they elicit an immune response. OspC down-regulation occurs during later stages of infection to avoid immune detection. Antibodies in the blood of vaccinated individuals bind to the Lyme disease organism during tick feeding and inactivate the spirochete before it can be transmitted to human hosts. However, in 2001 the vaccine was removed from the market because of unacceptable toxicity and side-effects (Pier et al., 2004). Immune system evasion by B. burgdorferi is executed by: (1) The down-regulation of immunogenic surface proteins by antigenic variation. OspA is down-regulated preventing an innate immune response, while OspC is upregulated to inactivate host complement proteins. (2) Inactivating host effector mechanisms: OspC proteins inactivate host complement (Sap15, Sap20, Ixodes scapularis anti-complement (ISAC) protein); expression of complement regulator-acquiring surface proteins prevent complement system activation once within the host (CD59-like protein) (Figure 3); IL-10 expression is induced within the host, causing the down-regulation of cytokine release and dampening immune response. CD59 proteins found in serum and cell membranes normally interfere with the terminal stage of complement activation (the assembly of the membrane attack complex, MAC). By mimicking CD59, B. burgdorferi prevents essentially prevents MAC formation. (3) Hiding within the extracellular matrix where phagocytosis is difficult for host immune cells: Degradation of extracellular matrix by plasminogen allows occupation of this niche, combined with upregulation of metalloproteinase-9 causing extracellular matrix degradation; B. burgdorferi can also attach to extracellular matrix proteins such as fibronectin. Surface proteins prevent opsonization, complement, and attack from mononuclear cells as the bacteria enters the host. Immediately after tick bite erythema (reddening of the skin) occurs at the site of entry. Figure 3. Borrelia burgdorferi produce inhibitors that interfere with complement activity. C9 protein is required for the formation of cellular pores initiated by the complement cascade. Identification and
Diagnosis: The infection can persist
even after antibodies for the bacteria are
developed by the host immune system, and are an
indicator of late stage infection. Antibody
screening is only effective if the patient has
no prior history of the disease or infection.
Blood testing for antibodies against B.
burgdorferi is effective within the first
month for patients exhibiting symptoms of the
disease such as a rash, tick bite, or stiffness
in muscles and joints. Serology is typically
carried out using enzyme-linked
immunosorbent assay (ELISA),
followed by Western blot, as the ELISA test for
antibodies against B. burgdorferi has a
high false positive rate due to Symptoms and Treatment: First signs of infection include erythema of skin (erythema migrans), typically occurring in a two-ringed target shape and can persist for two weeks to a month (Figure 4). Not all those affected develop a rash, but will often suffer fatigue, muscle and joint stiffness, and swollen lymph nodes often resembling flu like symptoms. Secondary skin lesions can also occur. As the redness of the rash fades, the bacteria are circulating throughout the body leading to a latent infection. If left untreated, infection can spread to joints, the heart, and the nervous system - a chronic infection. Latent infections include Lyme borreliosis and Lyme neuroborreliosis. Lyme borreliosis is associated with inflammation of joints (especially in the knees), tendons, and muscle occurs during latent infection. Meningitis and abnormal heart rhythms have also been noted. Lyme neuroborreliosis is a disorder of the central nervous system. This disease is linked with inflammation of nerve roots leading to piercing, radicular pain in the extremities, lymphocytic meningitis (inflammation of membranes surrounding lymph tissues), and neuritis located in the skull or peripheries. Chronic infection can result in muscle and nervous system damage, heart failure, and paralysis. Figure 4. This photograph depicts the pathognomonic erythematous rash in the pattern of a 'bull’s-eye', which manifested at the site of a tick bite on the posterior right upper arm of a woman who’d subsequently contracted Lyme disease. Treatment can be administered by antibiotics orally. Chronic persistent Lyme disease is treated for up to 28 days with antibiotics. If arthritis symptoms do not go away, a second two to four week course of antibiotics may sometimes be used. Antibiotics given by mouth (doxycycline, amoxicillin, or cefuroxime) are used most of the time. People with severe Lyme disease that affects the nervous system may receive two to four weeks of the antibiotic ceftriaxone via intravenous injections. Treating patients for longer periods of time is generally not thought to be helpful, even if symptoms do not go away. References: Pier, G.B., Lyczak, J.B., & Wetzler, L.M. (2004). Immunology, Infection, and Immunity. Washington: ASM Press. Rupprecht, T.A., Koedel, U., et al. (2008). The pathogenesis of Lyme neuroborreliosis: from infection to inflammation. Molecular Medicine, 14: 205-12. |