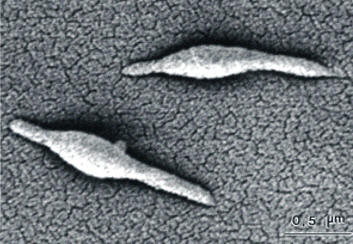
Mycoplasma pneumoniaeOverview: Mycoplasma pneumoniae is a round, pear-shaped, aerobic bacterium with the smallest known genome of any cellular organism containing 816 Kb (Kilobases), and a member of the Mycoplasmataceae family, of which 102 species affect humans and six affect animals (Figure 1). This organism ranges in size from 0.2 μm to 0.8 μm (micrometres) and can assume multiple morphologies, including a filamentous form. M. pneumoniae lacks a bacterial cell wall, but contains sterol-like molecules in their membranes for structural support; thus, the cell membrane is its outermost layer. Since it lacks a cell wall, the Gram staining technique cannot be applied to identify this pathogen, and that penicillin and other beta-lactam antibiotics are ineffective in treating infections. The lack of a cell wall also causes osmotic instability, but M. pneumoniae can survive in osmotically stable environments such as in a human host. Mycoplasma colonies grow by binary fission, forming a dense central core surrounded by a circular spreading area that is lighter than the core, resembling a 'fried-egg' on agar plates. Figure 1. This photomicrograph depicts two Mycoplasma pneumoniae bacteria. Clinical Infections: M. pneumoniae causes the respiratory infections tracheobronchitis (often accompanied by upper respiratory tract manifestations) and primary atypical pneumonia, also known as the ‘walking pneumonia’. Symptoms of infection can include fever, headache, aches and pains, chills, sore throat, nasal congestion, earache, malaise, and a persistent non-productive hacking cough. Symptoms can last for several weeks or months. Physical manifestations such as scattered rales and ronchi, throat infection, and cervical adenopathy can occur. It is important to note, however, that not all human hosts colonized by M. pneumoniae actually develop pneumonia, as one would assume due to the species name. M. pneumoniae is also thought to be involved in mediating other diseases and infections in its monopolization of the immune response. Some patients have developed severe bacterial and viral infections just after or during a M. pneumoniae infection. This is thought to occur by the creation of an environment that is anatomically, physiologically, and/or immunologically conducive to other organisms for invasion as well as for cellular damage. Pathogenicity: Evidence suggests that pneumonia caused by M. pneumoniae cycles through populations every four to five years. The life cycle of this organism can follow two paths. It reproduces either through binary fission or cell elongation followed by multiple cell divisions to form coccoid cells that are 0.2 μm to 0.3 μm in size. M. pneumoniae bacteria use a surface receptor to attach to host cell surfaces. This receptor can attach to a number of different cell types such as respiratory tract epithelia and red blood cells. At high concentrations, M. pneumoniae can inhibit ciliary action within the respiratory tract, as well as cause cell necrosis. This damage is caused by cytotoxins from M. pneumoniae, as well as indirectly from the host immune response. Virulence:
The virulence attributed to this bacteria is
correlated to the lipid-associated membrane
proteins that are exposed on the cell surface.
The expression of specialized polar tip
organelles for mediating attachment to host
cells is a coordinate interaction between
designated adhesins, interactive proteins, and
adherence-accessory proteins. By concentrating
adhesins at the tip of this structure,
Mycoplasma species are able to colonize
mucous membranes and eukaryotic cell surfaces.
In fact, the M. pneumoniae adhesin
sequences bear significant homology to mammalian
structural proteins (CD4+ on T helper
cells and MHC class II molecules). It is thought
that this homology is what provokes an antiself
response that leads to immune disorders. Transmission and Diagnosis: M. pneumoniae-mediated infection can be spread through close personal contact via respiratory droplets, and therefore confined populations such as schools and army barracks cause people to be at high risk. Since beta-lactams are useless in the treatment of infections caused by M. pneumoniae, the best treatment appears to be the body’s own immune system, especially the complement system via the alternative pathway. However, antibiotics tetracycline (adults only) and erythromycin can be used by people who wish to speed up healing. Unfortunately, there are no vaccines currently available. Diagnosis in the early stages is made on clinical grounds, but later stages require laboratory tests, such as complement fixations tests, which detects immunoglobulin G (IgG) and immumoglobulin M (IgM) specific for M. pneumoniae antigens, and enzyme-linked immunosorbent assay (ELISA). However, complement fixation lacks specificity and is unable to differentiate between the antibody classes, resulting in difficulty in differentiating acute from chronic or previous infections. IgM tests using ELISA can effectively indicate recent or current M. pneumoniae infection, especially in children. However, IgM levels are not always raised on infection or reinfection in adults. Hence, the separate detection of IgM and IgG by ELISA facilitates a more accurate diagnosis. An elevated titer of IgG of more than 1:80 is frequently interpreted as evidence of acute infection, whereas low levels of M. pneumoniae IgG can indicate either the early stage of acute infection or a past illness. Ideally, a second sample should be examined after two to three weeks, when a fourfold or greater increase of the IgG titer is interpreted as evidence of acute infection (Johnston & Martin, 2005). References: Johnston, S.L., & Martin, R.J. (2005). Chlamydophila pneumoniae and Mycoplasma pneumoniae: A Role in Asthma Pathogenesis? Pulmonary Perspective, 172: 1078–1089. |
|