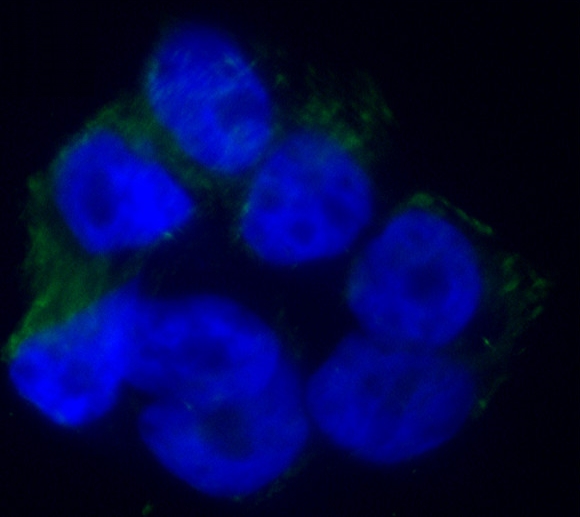
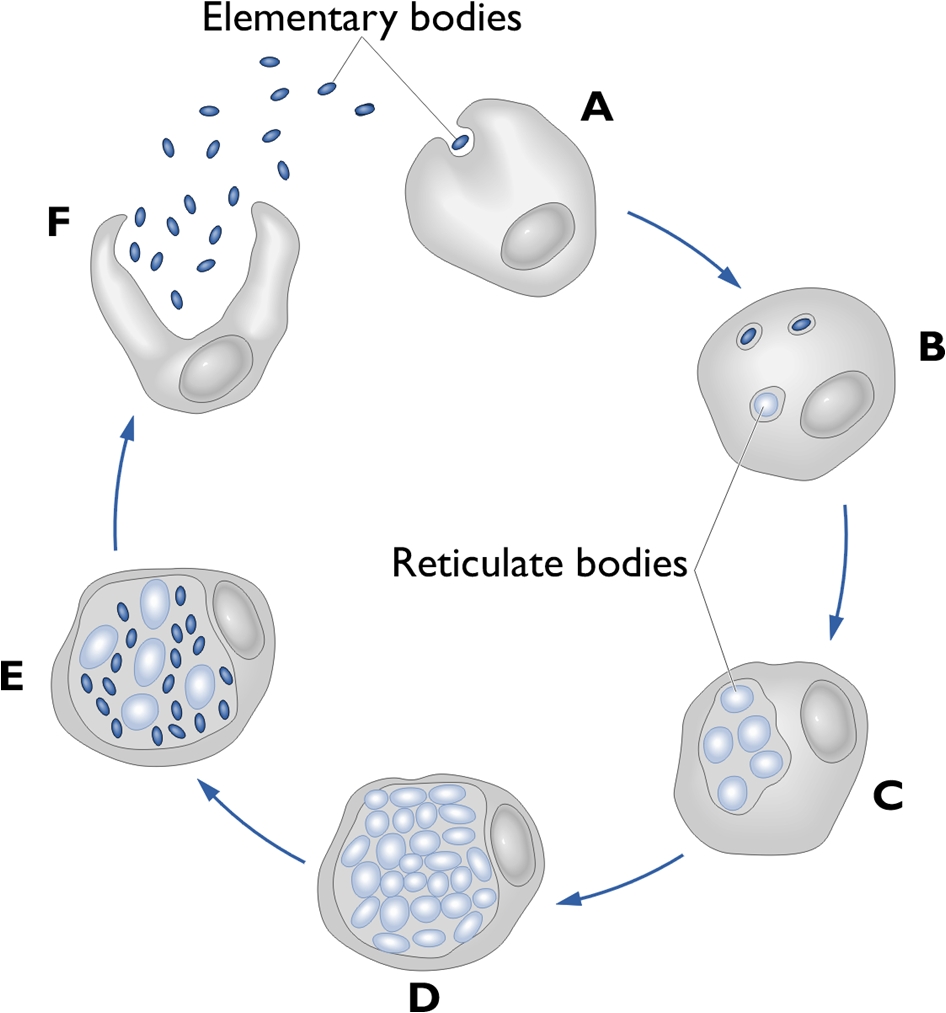
Chlamydia pneumoniaeOverview: Chlamydia pneumoniae is a Gram-Negative, non-motile, coccoid or rod-shaped, obligate intracellular bacterium that causes a plethora of illnesses in humans, and a member of the Chlamydiaceae family (Figure 1). Although classified as Gram-negative, C. pneumoniae bacteria do not possess a peptidoglycan layer. They have relatively low levels of phenotypic and genotypic variations in comparison to the other human pathogens within the Chlamydiaceae family (Burillo & Bouza, 2010). Figure 1. Annexin labeling of neruoblastoma cells infected with Chlamydia pneumoniae. Although C. pneumoniae is non-motile, studies have indicated that the pathogen's genome contains the following three flagellar protein orthologs: FliI, FlhA and FliF (Stone et al., 2010). The three orthologs are unable to produce a completely functional flagellar system; however, there is potential for the formation of a more basic flagellar system. C. pneumoniae bacteria are not classified as endospore-forming bacteria. During the developmental cycle, this species undergo rapid cellular replication within an inclusion body, which is then transported to the endoplasmic reticulum where fusion occurs with the exocytic vesicles (Rzomp et al. 2003). With respect to metabolism requirements, C. pneumoniae requires is oxygen to survive, but requires environments containing lower levels of oxygen than are present in the atmosphere (~20% concentration), thus it is microaerophilic. Enhanced replication and growth of the bacteria occurs as the level of oxygen increases within the environment (Juul et al., 2007). Life Cycle: Microscopic morphology studies indicate C. pneumoniae is involved in a developmental life cycle, in a sense that this species has a biphasic life cycle of two distinct physiological forms, beginning with an infectious, metabolically attenuated elementary body (EB) and a reticular body (RB) form (Figure 2). The elementary bodies, or survival form, are infectious and resilient, but lack metabolic activity. They have the ability to survive outside of human cells (Campbell & Kuo, 2004). Once conditions are suitable, attachment occurs and reticulate bodies are formed. The reticular bodies, or replicating form, actively divide within the host. They are non-infectious and metabolically active. Based on colonial morphology of C. pneumoniae, the elementary bodies are generally easy to identify due to their characteristic pear-shaped structure, containing a larger than normal amount of periplasmic space. Figure. The life cycle of Chlamydia pneumoniae. Pathogenicity: Chlamydial invasion is initiated by attachment of the EB to the host eukaryotic cell membrane and recruitment of actin to the site of attachment (Figure 2; A). This remodeling of the actin cytoskeleton is thought to be mediated by the type III secretion effector protein, and the translocated actin recruitment protein, which facilitates chlamydial entry into the host cell. Bacterial uptake involves modulation of the host extracellular signal-regulated kinase pathway, through the action of type III secretion effectors. Once internalized, the remainder of the chlamydial life cycle takes place within a membrane-bound vesicle known as an inclusion (Figure 2; B), where EBs differentiate into RBs, the non-infectious, metabolically active form (Stone et al., 2010) (Figure 2; C-D) Within the inclusion, RBs acquire amino acids, nucleotides, lipids and cholesterol from the host cell, events possibly orchestrated via type III secretion across the inclusion membrane, while at the same time inhibiting apoptosis of the host cell to ensure survival. Golgi fragmentation appears to be a crucial step in intercepting host pathways to obtain these nutrients and compounds, as well as in the maturation of the Chlamydiae species within the inclusion. The RB interacts with the inclusion membrane until such time as inclusion membrane RB docking sites are no longer available and an unknown signal triggers detachment of the RB from the inclusion membrane followed by asynchronous differentiation into EBs (Figure 2; E). The newly formed EBs then exit the cell by either cellular lysis or a packaged release mechanism termed extrusion (Stone et al., 2010) (Figure 2; F). Virulence: The following is a list of the different cells susceptible to the C. pneumoniae: Human epithelial cells (lungs), monocytes, macrophages, smooth muscle cells, and endothelial cells (Krull et al., 2006) C. pneumoniae possesses several key virulence factors. To begin, the intracellular parasite bacteria has the ability to enter and replicate the cellular machinery, resulting in a wide range of adverse effects within the host cell, including pro-inflammatory activation, cytokine release, and upregulation of adhesion molecules (Krull et al., 2006). The initial attachment of an EB to the host cell heparin sulfate-like glycosaminoglycans (GAG) receptor leads to the activation of a complex growth cycle involving C. pneumoniae. This promotes EB entry into the host (Krull et al. 2006). Additional virulence factors associated with C. pneumoniae include heat-shock protein 60 (cHsp60, GroEL-1), outer membrane protein–A (OmpA), and a type III secretion apparatus mentioned earlier (Krull et al., 2006). Identification: For diagnostic purposes, C. pneumoniae is typically identified by examining culture samples. The pathogen is an obligate intracellular organism; therefore, samples of C. pneumoniae should include host cells with the pathogen located within it. The microimmunofluroscence test (Figure 1) is used to diagnose acute C. pneumoniae infection by examining and identifying key phenotypic structures (Burillo & Bouza, 2010). Enzyme immunoassays (EIA) are also performed in order to detect the various immunoglobulin levels, which determine the specific pathogen present. Clinical Infections: Although individuals of all ages are at risk for contracting the pathogen, young children and adolescents are at highest risk. C. pneumoniae is commonly associated with the respiratory system, since the bacteria itself is transmitted from person-to-person via aerosolized respiratory secretions (Burillo & Bouza, 2010). The following is a list of complications are associated with C. pneumoniae, namely, atherosclerosis, Alzheimer’s disease, pharyngitis, bronchitis, arthritis, acquired-pneumonia, respiratory infection, asthma, and multiple sclerosis. C. pneumoniae is most commonly found over-populated, crowded environments, such as university campuses and military bases. Humans are the key reservoir, while C. pneumoniae has not been identified in animal reservoirs. References: Burillo, A., & Bouza, E. (2010). Chlamydophila pneumonia. Infectious Disease Clinics of North America, 24: 61-71. Campbell, L.A., & Kuo, C. (2004). Chlamydia pneumoniae – an infectious risk factor for atherosclerosis. Nature Reviews, 2: 23-32. Juul, N., Jensen, H., Hvid, M., Christiansen, G., & Birkelund, S. (2007). Characterization of in vitro chlamydial cultures in low-oxygen atmospheres. Journal of Bacteriology, 189: 6723-6726. Krull, M., Bockstaller, P., Wuppermann, F.N., Klucken, A.C., Muhling, J., Schmeck, B., Seybold, J., Walter, C., Maass, M., Rosseau, S., et al. (2006). Mechanisms of Chlamydophila pneumoniae-Mediated GM-CSF release in human bronchial epithelial cells. American Journal of Respiratory Cell and Molecular Biology, 34: 375-382. Stone, C.B., Bulir, D.C., et al. (2010). Interactions between flagellar and type III secretion proteins in Chlamydia pneumoniae. BMC Microbiology, 10(18): 1-12. |