Rickettsia prowazekiiRickettsia prowazekii is the pathogenic bacterium responsible for epidemic typhus fever, an infectious disease that has plagued humans ever since it was first recorded in Europe nearly 1000 years ago (Szybalski, 1999). More recently, R. prowazekii is estimated to have infected nearly 30 million humans following the First and Second World War (Andersson et al., 1998), and continues to pose a major health threat in certain parts of the world, despite the availability of antibiotics.
R.
prowazekii is typically transmitted to
humans by the body louse – an obligate
ectoparasitic arthropod (Figure 1) (Baxter,
1996). In its vegetative state, the pleomorphic
R.
prowazekii is 0.3 μm
(micrometres) to 0.5 μm by 0.8 μm to
2.0 μm in size (Walker, 1998), which makes this
species too small to be clearly seen under an
ordinary light microscope.
R.
prowazekii belongs to a large group of
Gram-negative bacteria known as
α-proteobacteria that multiply in eukaryotic
cells. This suggests
that they can only grow and reproduce within the
living cells of their host. Although classified
as Gram-negative bacteria,
R.
prowazekii are poorly stained by the Gram
method and are better visualized using the
Giemsa or Giménez stains
(Raoult
et al.,
2004). Figure 1. This image shows an adult female human body louse (Pediculus humanus humanus) along with two larval young.
Intracellular
R. prowazekii
often
appear as short, paired or single,
lanceolate-to-ovoid coccobacilli (Raoult
et al.,
2004). To undergo cellular respiration, this
species requires oxygen
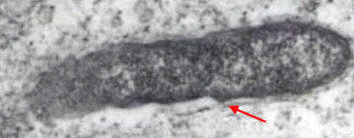
as a terminal electron acceptor. Its envelope
consists of three major layers: an innermost
cytoplasmic membrane, a thin electron-dense
rigid cell wall containing peptidoglycan, and an
outer layer, which contains lipopolysaccharide
endotoxin (Yu & Walker, 2005) and immunodominant
surface-exposed proteins that provide structure
or potentially contribute to host cell adhesion
or other host cell interactions (Figure 2)
(Raoult
et al.,
2004).
Cells of
R. prowazekii also possess intracytoplasmic
invaginations of the plasma membrane – a
morphological feature that resembles cristae
found in the mitochondria. Unlike the
mitochondria, however, members of this species
possess a flagellum for motility – a feature not
common among the family Rickettsiaceae (Yu &
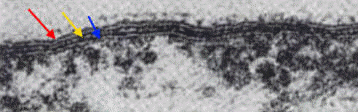
Walker, 2005). Figure 2. A transmission electron micrograph revealing the cell envelop of R. prowazekii. The red arrow points to the outer layer, the yellow arrow points to the thin electron-dense rigid cell wall, and the blue arrow points to the innermost cytoplasmic membrane [196 000 X].
Since R.
prowazekii requires an intracellular
habitat, cells must be co-cultivated in tissue
culture or yolk sac of developing chicken
embryos (Figure
3,
Figure
4); hence, a characteristic
colonial morphology is inexistent. Under poor
nutritional conditions, cells of
R.
prowazekii grow into long, 4 μm filaments
and cease to multiply via transverse binary
fission
(Yu
& Walker, 2005).
However, when proper conditions are restored,
they immediately divide into their ordinary
short rod form and engage in extensive movements
until released by the disruption of massively
infected cells.
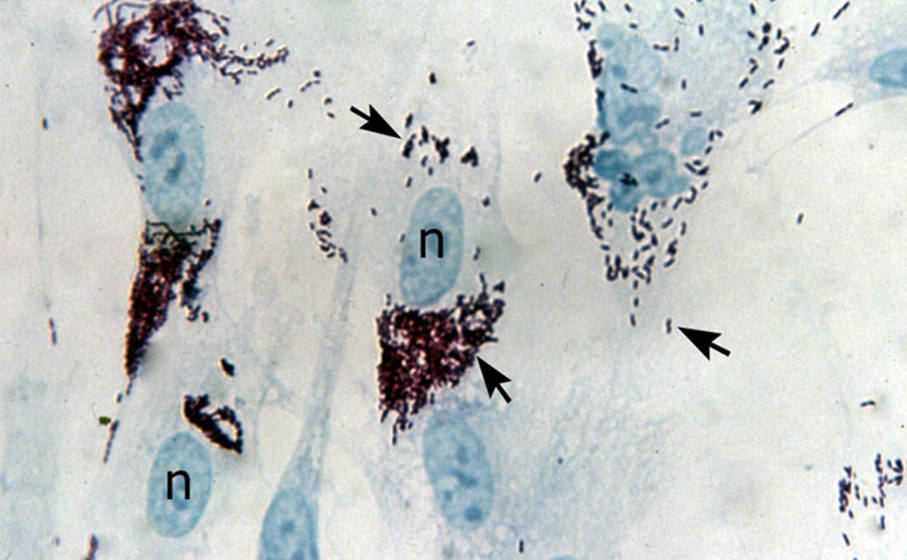
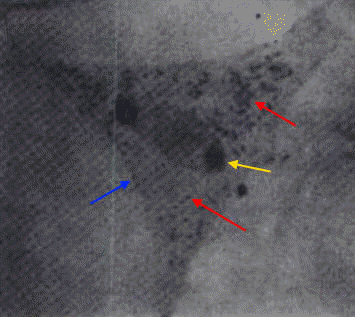
Figure 3. Rickettsia prowazekii bacteria growing inside human fibroblasts. The black arrows point to the bacteria and the 'n' indicates host cell nuclei. Figure 4. This transmission electron micrograph depicts a chicken embryo fibroblast infected with a large mass of R. prowazekii. The red arrows point to the bacteria, the blue arrow points to the fibroblast cell membrane, and the yellow arrow points to the nucleus of the fibroblast [1200 X]. The nutritional requirements of R. prowazekii, outside their host cell, are not known (Yu & Walker, 2005). What is known is that R. prowazekii acquires most of its amino acids from its host cell because only the genes associated with the biosynthesis of lysine and serine are present in its DNA (Yu & Walker, 2005). In addition, maximal growth of this species can only occur if there is a sufficient amount of host cell proline and serine or glycine (Yu & Walker, 2005). This implies that R. prowazekii has a highly permeable cytoplasmic membrane. Likewise, glycolytic intermediates and products, such as acetyl-CoA, must also be obtained by its host. Similar to the mitochondrion found in eukaryotic cells, this species produces its own physiological energy supply of ATP via the enzyme ATPase (Yu & Walker, 2005). This reason, along with the evidence suggesting that R. prowazekii shares a phylogenetic relationship with the mitochondria, is why some biologists speculate that this species may have given rise to the modern-day mitochondria. Altogether, to be able to identify this species for diagnostic purposes, samples containing bacterial cells must be cultivated in tissue culture at 35°C. Once nutrients are depleted, longer, more filamentous-like cells with prominent vacuoles will develop (Figure 5). Infected tissues stained with Giemsa should show bluish-purple organisms, whereas tissue stained with Giménez should show bright red organisms, with a decolorized background stain of pale greenish blue (Yu & Walker, 2005). Due to the inability to grow R. prowazekii on ordinary microbiological media, such as nutrient broth, diagnosis is often difficult (Ge et al., 2004). Figure 5. This transmission electron micrograph shows a large number of free cytoplasmic R. prowazekii late in chicken embryo fibroblast infection. The red arrow shows an example of a single vacuole-like structure that appears when nutrients are low or depleted, and the yellow arrow points to bacteria dividing by binary fission [13 600 X].
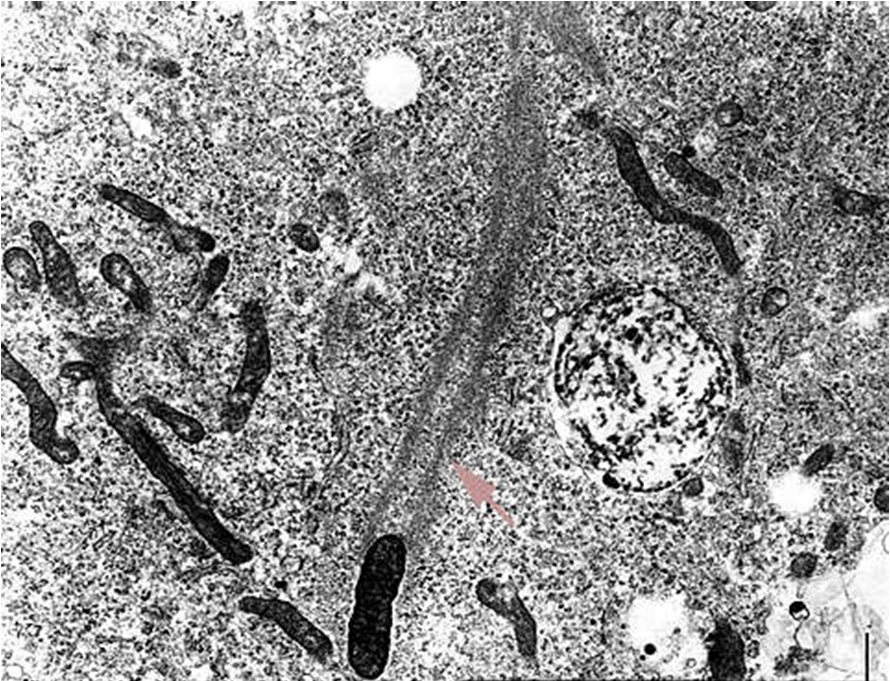
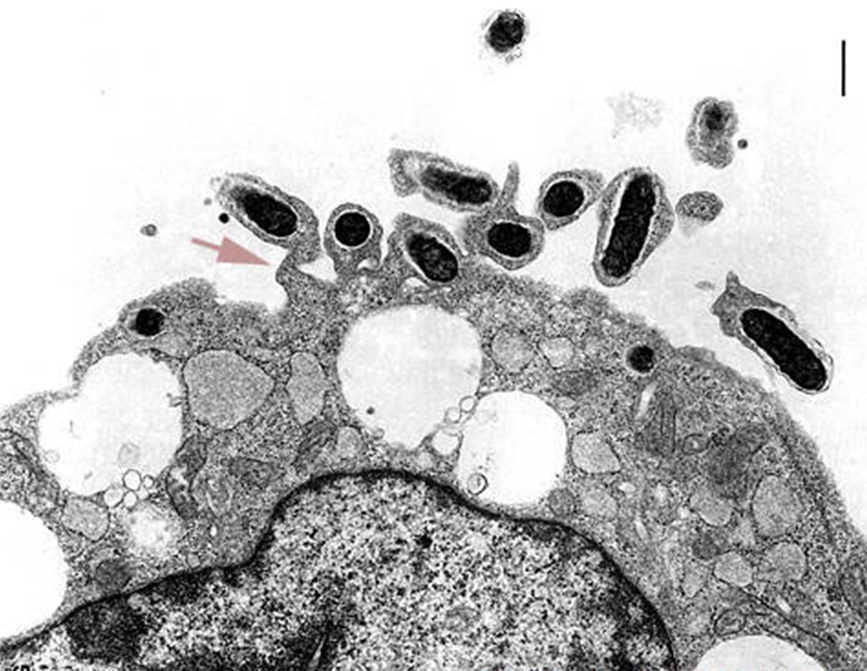
The entry of
R.
prowazekii into a human host cell (typically
a phagocyte) involves three steps: attachment
(via OmpA protein and a host cell receptor),
internalization and escape from the phagosome,
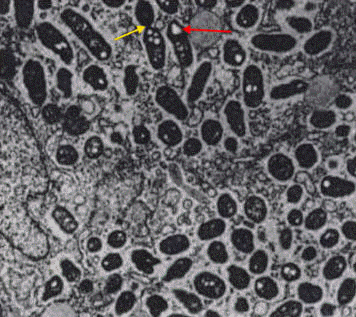
prior to lysosomal Figure 6. This micrograph shows R. prowazekii in the process of escaping phagosomal entrapment. The red arrow points to a break in the phagosome membrane [31 350 X].
Figure 7. Rickettsiae are propelled through the host cell cytoplasm. The area points to the polymerization of actin.
Figure 8.
Body louse (Pediculus humanus humanus), the flying squirrel flea (Orchopeas howardii), and louse (Neohematopinus sciuropteri) are the most effective vectors in the spread of R. prowazekii-induced epidemic typhus fever. Once transmitted to the mammalian host through a bite via arthropod saliva, the bacteria are found principally in the endothelium of blood vessels, particularly in those of the brain, skin and heart. This, in turn, causes hyperplasia of endothelial cells and localized thrombus formation, resulting in petechial rash, fever – which may reach 39°C – and terminal shock. Finally, areas of poor hygiene, such as refugee camps during a major famine or natural disaster, are places where diseases such as typhus fever tend to flourish. In fact, due to recent outbreaks in parts of Europe, Africa, and South America, some experts support the notion that epidemic typhus is a reemerging public health problem (Ge et al., 2004).
References
Andersson, G.E.,
Zomorodipour, A., Andersson, J.O.,
Sicheritz-Pontén, T., Alsmark, C.M., Podowski,
R.M., Näslund, K.A., Eriksson, A-S., Winkler,
H.H., & Kurland, C.G. (1998). The genome
sequence of
Rickettsia prowazekii and the origin of
mitochondria.
Nature,
396: 133-143.
Baxter, J.D. (1996). The Typhus Group.
Clinical
Dermatology, 14(3): 271-278.
Ge, H., Chuang, Y-Y.E., Zhao, S., Tong, M.,
Tsai, M-H., Temenak, J.J., Richards, A.L., &
Ching, W.M. (2004). Comparative Genomics of
Rickettsia prowazekii Madrid E and Breinl
Strains.
Journal of Bacteriology, 186(2): 556-565.
Raoult, D.,
Woodward, T., & Dumler, J.S. (2004). The history
of epidemic typhus. Infectious Disease
Clinics of North America,
18(1): 127-140.
Szybalski, W. (1999).
Maintenance of
human-fed live lice in the laboratory and
production of Weigl's exanthematous typhus
vaccine. In Maintenance of human,
animal, and plant pathogen vectors, K.
Maramorosch and F. Mahmood (eds.). Science
Publishers Inc., Enfield, New Hampshire,
161–179.
Walker, D.H. (1988). Pathology and pathogenesis
of the vasculotropic rickettsioses,
In:
D.H. Walker (Ed.),
Biology
of Rickettsial Disease, Florida: CRC Press
Inc. 115-138.
WHO: World Health Organization. (1976). Centers
for Disease Control and Prevention – Public
Health Image Library (CDC PHIL), I.D. # 5289.
Retrieved February 16th, 2010, from:
http://phil.cdc.gov/phil/details.asp.
Winkler, H.H., & Miller, E.T. (1982).
Phospholipase A and the Interaction of
Rickettsia prowazekii and Mouse Fibroblasts
(L-929 Cells).
Infection
& Immunity, 38(1): 109-113.
Yu, X-J., & Walker, D.H. (2005).
Genus I.
Rickettsia
da
Rocha-Lima 1916. In Bergey’s Manual of
Systematic Bacteriology, 2nd edn.,
vol. 2 (The
Proteobacteria). 96-106. Edited by Brenner
D.J., Krieg N.R., & Garrity, G.M., & Staley J.
T. New York: Springer.
|