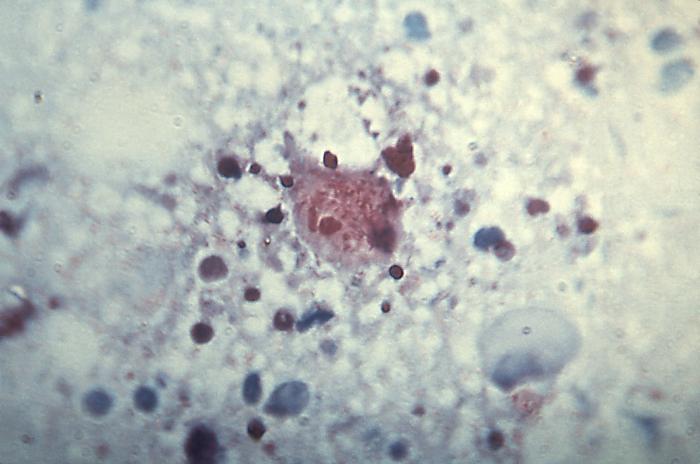
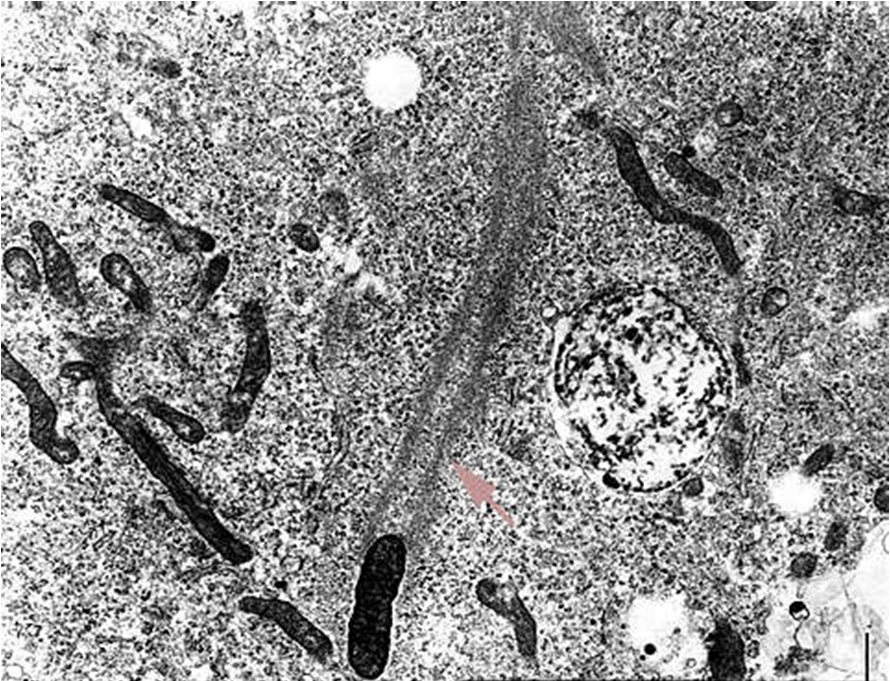
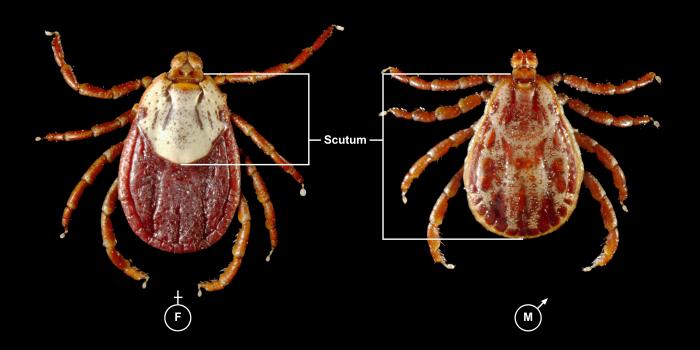
Rickettsia rickettsiaOverview: Rickettsia rickettsii is a Gram-negative, non-spore-forming, obligate intracellular aerobic bacterium, measuring 0.3 to 0.5 μm (micrometres) by 0.8 to 2.0 μm in size (Figure 1). R. rickettsii bacteria parasitize vascular endothelial cells in humans, causing Rocky Mountain spotted fever. The infection is transmitted by the bite of an infected tick while feeding on warm-blooded animals, including humans. Humans are accidental hosts in the R. rickettsii–tick life cycle and are not required to maintain the organism in nature. Figure 1. This micrograph reveals the presence of intracellular Rocky Mountain spotted fever bacteria, Rickettsia rickettsii. Spotted fever group Rickettsiae can grow in the nucleus or in the cytoplasm of the host cell. Once inside the host the Rickettsiae multiply, resulting in damage and death of these cells. R. rickettsii do not have flagella. Rather, the bacterium is propelled through the cytoplasm and into neighboring cells by stimulating the polymerization of host cell F-actin (Figure 2). R. rickettsii belongs to a large group of bacteria known as α-proteobacteria that multiply in eukaryotic cells. This suggests that they can only grow and reproduce within the living cells of their host. Moreover, this organism is able to metabolize glutamate, derived from host cells, via the citric acid cycle and aerobic respiration (Brenner et al., 2005). Figure 2. Rickettsiae are propelled through the host cell cytoplasm. The arrow points to the polymerization of actin. Identification: Diagnosis is made difficult by the germ's small size. R. rickettsii is not visible in blood smears, nor is it visible with conventional staining methods. Diagnosis usually relies on a combination of laboratory findings, physician findings and epidemiology. The best diagnostic test for R. rickettsii infection is serologic assays. Indirect immunofluorescence assay is typically used to detect immunoglobulin G (IgG) or immunoglobulin M (IgM) antibodies. After the first week of illness, patients usually have increased levels of IgM. IgG antibody levels typically appear between seven to ten days following the infection. The rising antibody level between IgG and IgM is a determinant of infection. Other assays include enzyme-linked immunosorbent assay and dot immunoassays. R. rickettsii bacteria are inoculated into human skin by ixodid (hard) ticks (Figure 3). There are two major R. rickettsii vectors in North America are the American dog tick and the Rocky Mountain wood tick. These ticks have four stages in their life cycle: egg, larva, nymph, and adult. After the eggs hatch, each stage must feed once to develop into the next stage. Both male and female ticks will bite. Rickettsiae are transmitted to a vertebrate host through saliva while a tick is feeding. It usually takes several hours of attachment and feeding, before the rickettsiae are transmitted to the host. The risk of exposure to a tick carrying R. rickettsii is low. In general, about 1% to 3% of the tick population carries R. rickettsii, even in areas where the majority of human cases are reported. Figure 3. This photograph depicts a dorsal view of both a female (left), and male (right) Rocky Mountain wood tick, Dermacentor andersoni. Note the smaller size of the female’s scutum compared to the male’s larger scutum. From the Latin word for “shield”, the scutum (dorsal shield), covers only a small part of the female’s dorsal surface, thereby, enabling her abdomen to expand, becoming engorged during feeding. The male’s scutum covers his entire dorsal surface, and is mottled with brown markings overall, while the female’s small scutum sports an almost entirely a cream-colored gray surface. Click to enlarge. Once inside the
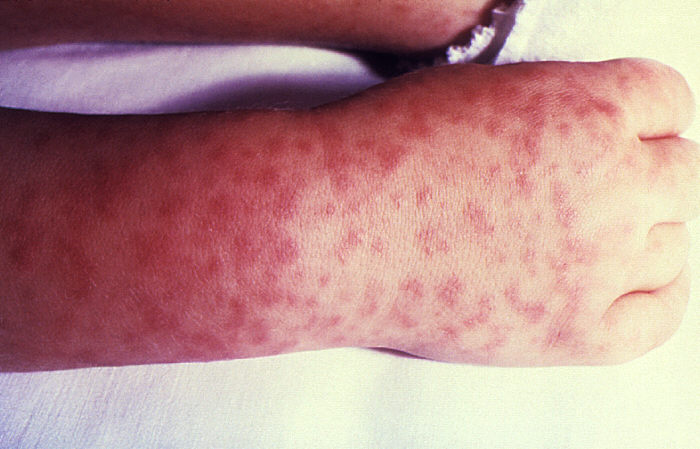
host, R. rickettsii attaches to
endothelial cells using rickettsial outer
membrane protein A (rOmpA). After Figure 4. Child's right hand and wrist displaying the characteristic spotted rash of Rocky Mountain spotted fever. Symptoms: Symptoms of Rocky Mountain spotted fever include fever, nausea, vomiting, severe headache, muscle pain, loss of appetite, spotted rash (wrists, forearms, ankles, palms and soles), abdominal pain, joint pain, diarrhea, low platelet count, low sodium level in the blood, elevated liver enzymes. Those who are immunocompromised (people with AIDS or cancer) are more likely to develop severe disease symptoms, and mortality rates are higher in people who are glucose-6-phosphate dehydrogenase-deficient. Treatment: Appropriate antibiotic treatment is initiated immediately when there is a suspicion of Rocky Mountain spotted fever on the basis of clinical and epidemiological findings. Treatment should not be delayed until laboratory confirmation is obtained. In fact, failure to respond to a tetracycline antibiotic argues against a diagnosis of Rocky Mountain spotted fever. Severely ill patients may require longer periods before their fever resolves, especially if they have experienced damage to multiple organ systems. Doxycycline (for adults at 100 mg every 12 hours or for children under 45 kg at 4 mg/kg body weight per day in two divided doses) is the drug of choice for patients with Rocky Mountain spotted fever. Therapy is continued for at least three days after fever subsides and until there is unequivocal evidence of clinical improvement, generally for a minimum total course of five to ten days. Severe or complicated disease may require longer treatment courses. Chloramphenicol is an alternative drug that can be used to treat Rocky Mountain spotted fever; however, this drug may be associated with a wide range of side effects and may require careful monitoring of blood levels, since it can cause aplastic anemia. References: Brenner, D., Krieg, N., Garrity, G., & Staley, J. (2005). Bergey’s Manual of Systematic Bacteriology Volume Two: The Proteobacteria (Part C). USA: Williams & Wilkins. |