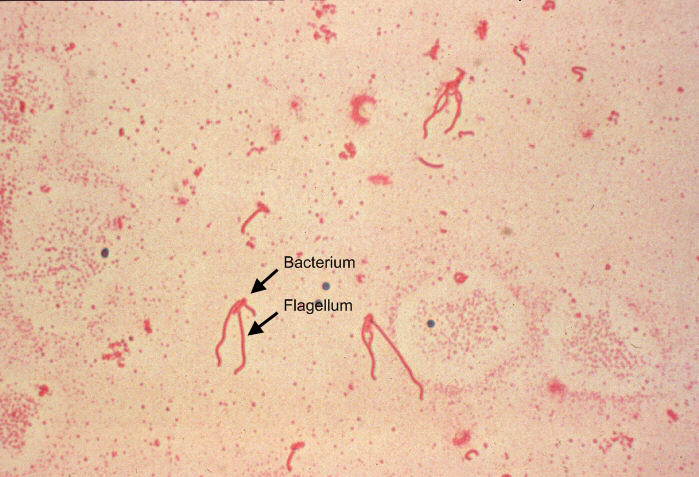
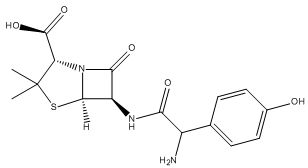
Yersinia enterocoliticaYersinia enterocolitica is a Gram-negative, asporogenous, rod-shaped bacterium and a member of the Enterobacteriaceae family of Proteobacteria that causes a broad range of gastrointestinal syndromes ranging from acute enteritis and enterocolitis to mesenteric lymphadenitis (Figure 1). Since Y. enterocolitica predominantly causes zoonotic infections, it is commonly found in swine, cattle, sheep, horses, dogs, cats, and rodents (De Berardis et al., 2004). Humans may be exposed by consuming contaminated foods, specifically undercooked pork products or unpasteurized milk (Trosky et al., 2008). Additionally, agricultural runoff may contaminate drinking water and spread to humans if ingested. Transmission may also occur through transfusion of contaminated blood. However, in a large number of human cases, the route of exposure generally remains unknown. Figure 1. A photomicrograph of Yersinia enterocolitica using Flagella staining technique. Approximately one week after infection, symptoms develop in the form of bloody diarrhea, fever and abdominal pain (De Berardis et al., 2004). These symptoms usually last for one to three weeks, and can be characteristic of a number of gastrointestinal diseases, including mesenteric lymphadenitis, enteritis, and terminal ileitis (Trulzsch et al., 2007). Due to the similarities in symptoms, mesenteric lymphadenitis is often misdiagnosed as acute appendicitis. Further complications may arise with the spontaneous manifestation of monarticular arthritis, which may occur one to six months after the initial infection (De Berardis et al., 2004). There are a few factors which
may increase or worsen the incidence of Y.
enterocolitica infection. Young children
demonstrate increased rates of infection
resulting in diarrhea and abdominal pain,
whereas the elderly are more susceptible to
developing monarticular arthritis. Since Y.
enterocolitica Diagnosis of a Y. enterocolitica infection may occur via stool or blood cultures. Y. enterocolitica grows well on most enteric media, however, most commonly stool samples are incubated at 25°C on Cefsulodin-irgasannovobiocin (CIN) agar for 18 to 20 hours (Bottone, 1997). This media is highly selective for Y. enterocolitica, and a positive diagnosis can be seen with the growth of small colonies with red centres. The detection of bacteria in bloodmay occur via an ELISA specific for IgA, IgG and IgM antibodies produced in the presence of Y. enterocolitica (Bottone, 1997). Although there is no vaccine
available to date, aggressive antibiotic therapy
is effective at eliminating Y.
enterocolitica post-infection (De Berardis
et al., 2004). Common antibiotics
include ampicillin, gentamicin, criprofloxacin,
amoxicillin (Figure 2), cefuroxime, imipenem, and
cefoperazone (Bottone, 1997). Figure 2. Chemical structure of amoxicillin. The oral infective dose of
Y. enterocolitica has been found to be
108 to 109 organisms/mL (De Berardis et al.,
2004). From this small culture, the bacteria are
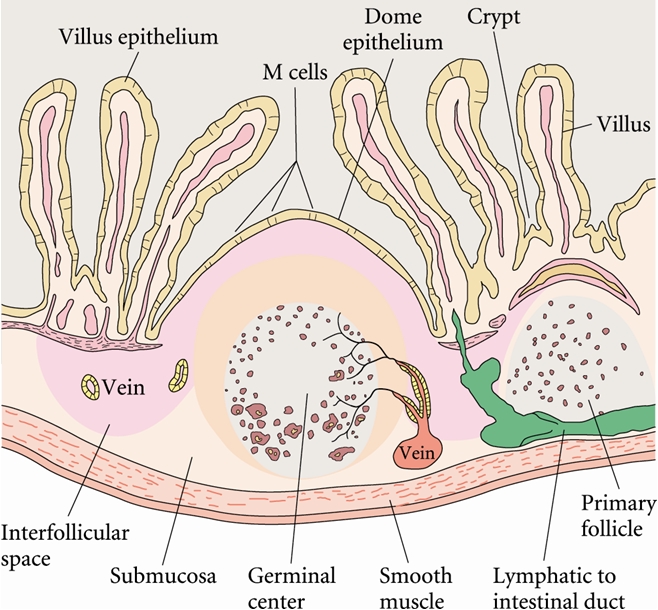
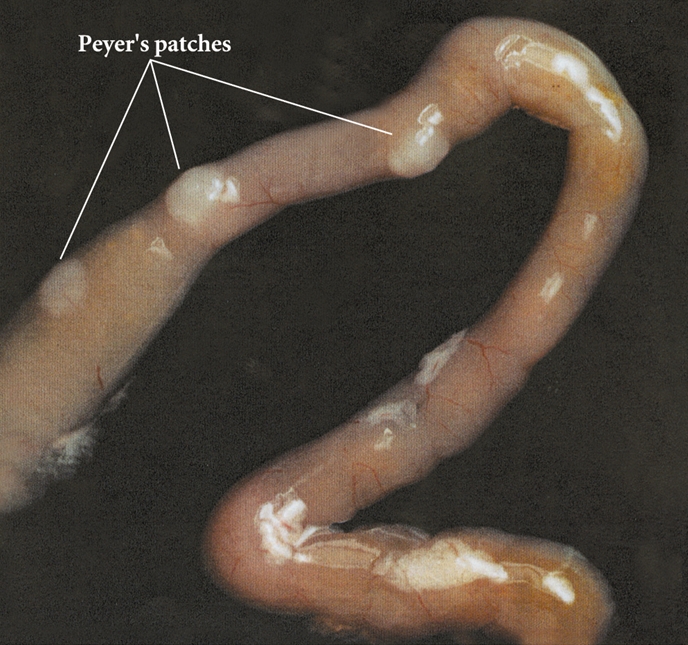
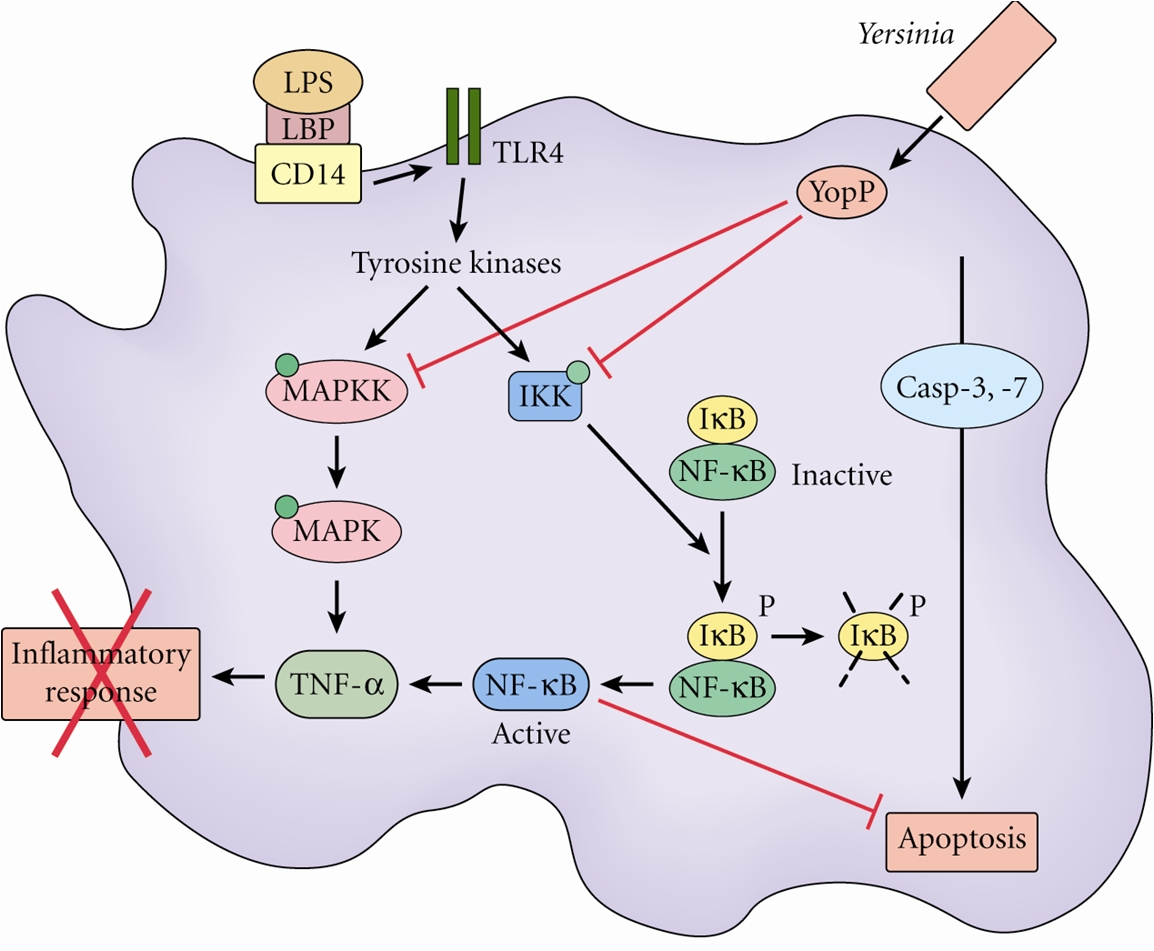
able to proliferate To overcome the mucosal barrier in the small intestine, Y. enterocolitica utilize M cells for translocation (Figure 3). This is achieved through the exploitation of Invasin (Inv) and Yersinia adhesion A (YadA). Invasin facilitates adhesion to β1 integrins, which are expressed on M cells (Roppenser et al., 2009). Further adhesion occurs through extracellular matrix bridging between YadA, collagen and fibronectin (Oellerich et al., 2007). Together, these virulence factors allow Y. enterocolitica to permeate the intestinal cells, where the bacteria can then invade the Peyer’s patches, or spread systemically to the spleen, liver, or mesenteric lymph nodes (Wiedig et al., 2005) (Figure 4). Abscess formation in the Peyer’s patches, spleen and liver are due to the presence of a single bacterium. This bacterium may become lodged in the capillary vessels, and upon replication cause a monoclonal abscess (Oellerich et al., 2007). Figure 3. Antigens transported across the intestinal epithelium by M cells are delivered to the Peyer's patches. Click to enlarge. Figure 4. The Peyer's patches are visible as a small bulge in the side of the small intestine. Y. enterocolitica possess a 70 kb DNA plasmid, which is essential to its virulence. This plasmid encodes a Type III secretion system (T3SS) and effectors known as Yersinia outer proteins (Yops). The T3SS consists of a base structure, and a needle-like protein used for the injection of effectors into the target cell (Koberie et al., 2009). Y. enterocolitica contain six Yops (YopH, YopE, YopO, YopM, YopJ and YopT) which become activated once inside the target cell. YopH, YopT, YopO and YopE are capable of disrupting the formation of actin microfilaments among macrophages and neutrophils. This prevents the phagocytosis of the bacterium. YopE is also important for regulating the amount of effectors injected into the target cells (Trosky et al., 2008). YopM does not contain catalytic activity, but acts as a scaffold protein once in the nucleus. Finally, the function of YopJ is to increase programmed cell death in the infected cells, thereby suppressing the immune system and prolonging the survival of the bacterium (Koberie et al., 2009). A summary of the effects of YOPs are illustrated below; take note on how YopP blocks the phosphorylation cascades (MAPK & NFkB pathways) and TNFα production, causing apoptosis to occur via the caspase pathway (Figure 5). Figure 5. Effects of Yersinia YOPs on inhibition of the inflammatory response needed to resist infection. Click to enlarge. The injection of effectors into the target cells induces the MHC class I pathway, as intracellular antigens are present. This in turn induces CD8+ T cell responses (Wiedig et al., 2005). Also, the complement cascade may become activated in the presence of LPS found in the outer membrane of these Gram-negative bacteria. However, Y. enterocolitica are able to efficiently resist complement and opsonization by utilizing the bacterial outer proteins YopA and Ail (Kirjavainen et al., 2008). The unique ability of Y. enterocolitica to effectively suppress the immune system has contributed to its increased virulence within the human body. References: Bottone, E.J. (1977). Yersinia enterocolitica: The Charisma Continues. Clinical Microbiology Reviews. 10(2), 257-276. De Berardis, B., Torresini,
G., Brucchi, M., Marinelli, S., Mattucci, S.,
Schietroma, M., Vecchio, Kirjavainen, V., Jarva, H.,
Biedzka-Sarek, M., Blom, A.M., Skurnik, M., &
Meri, S. (2008). Yersinia enterocolitica Serum
Resistance Proteins YadA and Ail Bind the
Complement Oellerich, M.F., Jacobi,
C.A., Freund, S., Niedung, K., Bach, A.,
Heesemann, J., & Trulzsch, K. (2007). Yersinia
enterocolitica Infection of Mice Reveals Clonal
Invasion and Abscess Roppenser, B., Roder, A., Hentschke, M., Ruckdeschel, K., & Aepfelbacher, M. (2009). Yersinia enterocolitica differentially modulates RhoG activity in host cells. Journal of Cell Science. 122(5), 696-705. Trosky, J.E., Liverman,
A.D.B., & Orth, K. (2008). Yersinia outer
proteins: Yops. Cellular Microbiology. 10(3),
557-565. Wiedig, C.A., Kramer, U., Garbom, S., Wolf-Watz, H., & Autenrieth, I.B. (2005). Induction of CD8+ T cell responses by Yersinia vaccine carrier strains. Vaccine. 23(42), 4984-4998. |