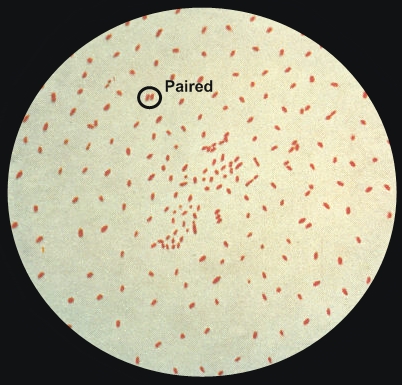
Bordetella pertussisOverview: Bordella pertussis is a Gram-negative, coccobacillus-shaped, aerobic bacterium, belonging to the family Alcaligenaceae. Like other Gram-negative species, B. pertussis possesses lipooligosaccharide (LOS, or endotoxin) in its cell-wall outer membrane. Unlike the typical lipopolysaccharide found in most species of Gram-negative bacteria, this LOS contains lipid A and an oligosaccharide layer, which is used to trigger the induction of excessive interferon production, mitogenicity, and polyclonal activation of B lymphocytes. In addition, when B. pertussis is grown on a medium, it is typically found at random and independent of each other or in pairs (Figure 1). Sheep blood is the preferred medium used to grow the bacteria, but a synthetic medium of amino acids, buffer solution, and growth factors can also be used as an alternative. B. pertussis is non motile and is the causative agent of the whooping cough.
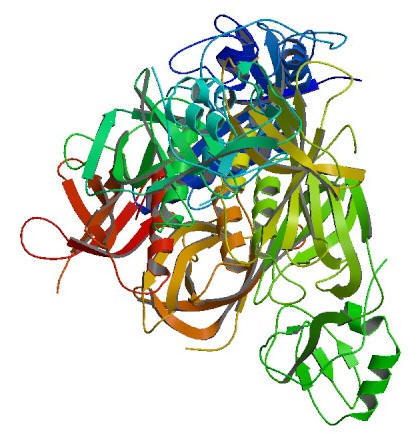
Figure 1. A photomicrograph of Bordetella pertussis bacteria using Gram stain technique. Virulence and Pathogenicity: Aside from LOS, another major virulence factor of B. pertussis is pertussis toxin (PT) (Figure 2). PT is a single protein that has a wide array of pathogenic activity and is produced only by B. pertussis; that is, no other species of the Bordetella genus is capable of producing PT. The B oligomeric (a heteropentameric moiety of the PT protein) is responsible for binding of the toxin to susceptible host cells and transporting the 'A protomer' across the eukaryotic cell membrane. Likewise, PT also functions as an adhesion in its ability to bind to ciliated cells of the respiratory tract and alveolar macrophages. Interestingly, binding of filamentous hemagglutinin (another virulence factor associated with B. pertussis) to macrophages leads to phagocytosis of the organisms without oxidative burst associated with phagocytosis, thus allowing the survival of the bacteria inside host immune cells. Furthermore, the enzymatic A subunit of the PT has adenosine diphosphate ribosyltransferase activity. This enzyme transfers ADP-ribose groups from NAD to the Hi (inhibitory) regulatory proteins that normally function in cell signal transduction, thus preventing the exchange of GDP for GTP - a deactivating process. This biochemical mechanism is believed to be responsible for many of the clinical signs and symptoms of pertussis, including the characteristic whooping cough. PT is the only molecule that is represented in every pertussis vaccine formulated thus far.
Figure 2. The crystal structure of pertussis toxin. Clinical Infections: When B. pertussis enters the respiratory tract and starts colonizing, a large buildup of bacteria can readily be recovered from the pharynx, allowing for quickly determination whether the patient's abnormal cough is caused by this pathogen. At this point, antibiotics would be administered to treat the individual's illness and prevent a more serious health problem. In more severe cases, B. pertussis infections have been shown to cause dehydration, loss of appetite, and even seizures. In order to supplement the effects of the antibiotics, increasing fluid intake and enhancing hydration status is recommended, since it helps to flush out the bacteria or toxins that are causing the inflammation. Depending on the age of the infected individual, the infection can range from mild and requiring no treatment, such as in adults, to potentially serious when it comes to infants. B. pertussis is typically transmitted from person-to-person, and was at one point one of the most frequent diseases before vaccination was introduced. Since most people are vaccinated at an early age in life, the only group of individuals at risk are those who are unvaccinated and become infected with the bacteria. Treatment: Treatment with an effective antibiotic (erythromycin or azithromycin) shortens the infectious period, but does not generally alter the outcome of the disease; however, when treatment is initiated during the catarrhal stage, symptoms may be less severe. Three macrolides (erythromycin, azithromycin and clarithromycin) are used in the United States for treatment of B. pertussis infection; trimethoprim-sulfamethoxazole is generally used when a macrolide is ineffective or is contraindicated. Close contacts who receive appropriate antibiotics (chemoprophylaxis) during the 7 to 21 day incubation period may be protected from developing symptomatic disease. Close contacts are defined as anyone coming into contact with the respiratory secretions of an infected person in the 21 days before or after the infected person's cough began. There is no known antitoxin. |