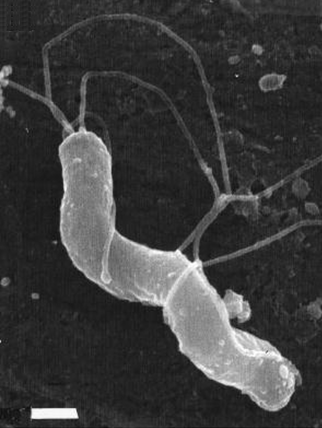
Helicobacter pyloriHelicobacter pylori is a Gram-negative, curved S-shaped bacterium, which also has a non-cultural coccoid form with rounded ends (Figure 1). The species name stems from: Helico, meaning helical or spiral shape, bacter, meaning bacteria, and pylori, refering to pylorus region of the stomach. This pathogen possesses five to seven distinct flagella and grows best when cultured at 37°C (Figure 2). Interestingly, in resting individuals, 37°C is the stomach's temperature - the targeted organ; however, the bacteria is able to withstand temperatures as low as 25°C.
Figure 1. A computer-generated image depicting a Helicobacter pylori bacterium.
Figure 2. S-shaped Helicobacter pylori with five to seven sheathed polar flagella [bar = 0.5 μm]. Colonies are non-pigmented (possess no particular colour), translucent, and about one to two millimeters in diameter. H. pylori is also microaerophilic (requiring low oxygen levels to survive) and can be identified metabolically since this species is catalase positive, oxidase positive, and urease positive. H. pylori adapts to the acidic environment of the stomach by hydrolyzing (chemically breaking down a molecule with H2O) urea rapidly, buffering the acidic environment, which can lead to peptic ulcers. Prior to its discovery, people believed that stress was the main cause of ulcers. Although stress is capable of potentially decreasing one's energy level and immune response, stress should only be consider a contributing factor to a particular problem and not the main cause. H. pylori can be
transmitted by fecal-oral spread and is rarely
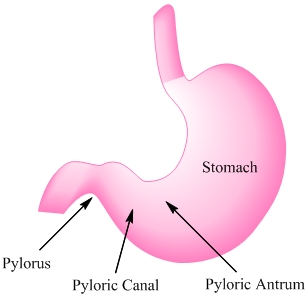
Figure 3. Regions of the stomach affected by Helicobacter pylori.
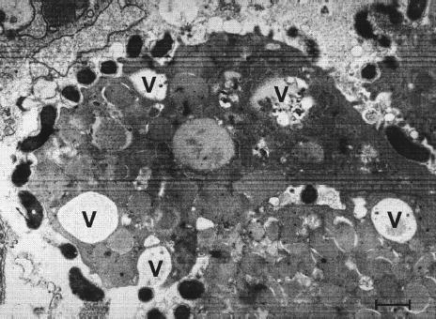
Figure 4. Intracytoplasmatic vacuoles (V) in gastric epithelial cells induced in vivo by Helicobacter pylori [bar = 0.5 μm]. Individuals infected with H. pylori never experience clinical symptoms other than chronic gastritis. However, symptoms of H. pylori infection include dyspeptic symptoms, such as inflammation (gastritis), weight loss, cramps, bad breath, burping, nausea, vomiting, flatulence (farting), black and tarry stools with foul odour, and loss of appetite. Diagnostic tests utilized to characterize H. pylori infection include a urease enzyme test where the infected person drinks a carbon labeled urea solution in which the bacteria can metabolize. The labeled carbon dioxide can then be detected in the breath. Another test includes a biopsy through endoscopy. The histology of the biopsy sample is analyzed for the pathogen' presence and to determine the degree of infection and damage. Other non-invasive tests include a non-quantitative enzyme-linked immunosorbent assay (ELISA) which detects H. pylori antibodies in blood serum, and by a fecal antigen test. Treatment includes antibiotics to kill the bacteria, H2 receptor antagonists (reduce stomach acid to prevent irritation), and proton-pump inhibitors to reduce the amount of stomach acid, and surgery to treat developing ulcers. Risk factors that have been shown to be related with H. pylori infection include smoking, those who are occupationally exposed to wastewater, poor hygiene practices, density or crowding in a population, and family history of gastric disease. References: Blaser, M., & Atherton, J. (2004). Helicobacter pylori persistence: biology and disease. Clinical Investigation, 113: 321-333. Brown, L. (2000). Helicobacter pylori: Epidemiology and Routes of Transmission. Epidemiology review, 22: 283-297. Malfertheiner, P., Bornschein, J., & Selgrad, M. (2010). Role of Helicobacter pylori infection in gastric cancer pathogenesis: A chance for prevention. Digestive Diseases, 11: 2-11. |