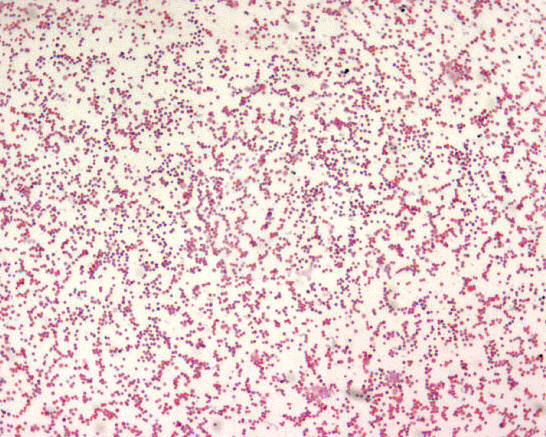
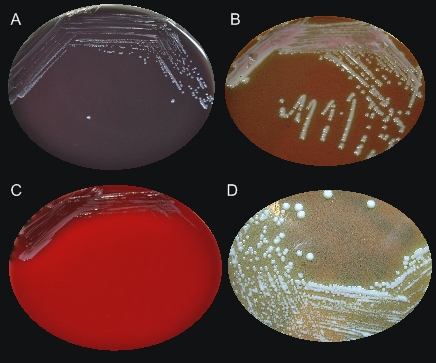
Francisella tularensisOverview: Francisella tularensis is a poorly staining, Gram-negative, non-motile coccobacillus bacterium, ranging from 0.2 by 0.2 to 0.7 µm in size (Figure 1). This species is highly infectious, causing the plague-like disease tularemia (also called glandular fever, rabbit fever, tick fever, and deer fly fever) in animals and humans. Typically, people become infected through the bite of infected insects (most commonly, ticks and deerflies), by handling infected sick or dead animals (most commonly, rabbits, rodents, and hares), by eating or drinking contaminated food or water, or by inhaling airborne bacteria [↓]. Generally, F. tularensis is strictly aerobic and may require up to three days before growth is detected in culture (Murray et al., 2009).Figure 1. Francisella tularensis is gram-negative in its staining morphology. Identification/Colony Characteristics: When cultured on nutrient medium, F. tularensis bacteria are slow-growing and have specific requirements for iron and cysteine or cystine. This species can be cultured on various media, such as sheep blood agar, MacConkey II agar, chocolate II agar, modified Thayer-Martin agar, buffered charcoal yeast extract, thioglycollate broth, or cysteine heart agar (Figure 2). Bacteria grown on cysteine heart agar medium produce smooth, greenish-white colonies 2 to 4 millimetres in length, with an opalescent sheen, 24 to 72 hours after cultration (Figure 2-D). In addition, F. tularensis is oxidase-negative, urease-negative, and β-lactamase positive. Figure 2. Colonization of Francisella tularensis on various media, namely: (A) buffered charcoal yeast extract; (B) chocolate agar medium; (C) sheep’s blood agar; (D) cysteine heart agar. Pathogenicity: F. tularensis has two main pathogenic serotypes: Subspecies tularenis (type-A) and subspecies holarctica (type-B). Both types possess a thin, antiphagocytic lipid capsule of high lipid concentration, and the lose of the capsule is associated with decreased virulence. This pathogen evades killing by cells of the innate immune system such as macrophages by utilizing genes encoding a pathogenicity island, iglABCD. The iglABCD operon is responsible for intracellular growth. It does this by preventing the endosome or phagosome (both terms are synonymous) from becoming acidified (Clemens et al., 2004). Recall that once the bacteria is ingested by a phagocyte, the initially non-acidified endosome (a vacuole that contains the ingested matter of a phagocytic cell) becomes acidified; bacteria are unable to survive in an acidic environment. F. tularensis disrupts the phagosomal membrane so that its morphologically gets disrupted, preventing acidification and allowing the bacteria to gain direct access to the macrophagic cytoplasm (Clemens et al., 2004). It then utilizes these cells as a niche for replication and dissemination to other organs within the host. Subsequently, an accumulation of macrophages without the removal of bacteria initiates the formation of granulomas, which form to wall off areas of infection and inflammation. Host cell death occurs as a result of septic shock, which occurs due to large amounts of cytokine release. The pathogen's mode of infection is summarized in Figure 3. Figure 3. Francisella tularensis enters the respiratory tract and (2) the lamina propria of the respiratory bronchioles via M cells; (3) Digested antigen is taken up by dendritic cells; the dendritic cells travel to regional lymph nodes and present F. tularensis antigens to T-helper 1 cells; (4) T-helper 1 cells proliferate; they may return to site of initial infection; (5) restimulation by local antigen presenting cells results in interferon-γ production and macrophage activation; (6) Failure to clear the F. tularensis results in granuloma formation. Interestingly, It has been noted that a strong innate response involving interferon-γ (INF-γ) and tumor necrosis factor (TNF) are important for controlling bacterial replication in macrophages in the early stages of infection (Murray et al., 2009). This makes sense because recall that these two cytokines (INF-γ and TNF) are normally responsible for activating macrophages to produce reactive oxygenated species and nitric oxide, which are highly toxic to bacteria. The humoral response (production of antibodies) is less important for elimination of this facultative intracellular pathogen. Clinical Diseases: F. tularensis subspecies exhibit a broad host ranges. Diseases caused by this species is subdivided into several forms, based on clinical presentation, namely: typhoidal (systemic signs of sepsis), pneumonic (pulmonary symptoms), oculoglandular (eye involvement and swollen cervical lymph nodes), pharyngeal, ulceroglandular (cutaneous ulcer and swollen lymph nodes), and glandular (primarily swollen lymph nodes with no other localized symptoms) (Murray et al., 2009). The incubation period is roughly three to five days and the sign and symptoms people develop depends on how they are exposed to tuleremia. Possible symptoms include skin ulcers, swollen and painful lymph glands, inflamed eyes, sore throat, mouth sores, diarrhea or pneumonia. If the bacteria are inhaled, symptoms can include abrupt onset of fever (39.7°C), chills, headache, muscle aches, joint pain, dry cough, and progressive weakness. Abdominal sonogram of infected individuals can show small pockets of ascites in the abdomen and pelvis (accumulation of serous fluid in the peritoneal cavity), containing neutrophils, lymphocytes and macrophages. People with pneumonia can develop chest pain, difficulty breathing, bloody sputum, and respiratory failure. Tularemia can be fatal if the person is not treated with appropriate antibiotics, such as streptomycin or gentamycin. However, getting vaccinated is a better way to prevent an infection caused by this species. References: Clemens, D.L., Lee, B.Y., & Horwitz, M.A. (2004). Virulent and avirulent strains of Francisella tularensis prevent acidification and maturation of their phagosomes and escape into the cytoplasm in human macrophages. Infection and Immunity, 72(6): 3204-3217. Murray, P.R., Rosenthal, K.S., & Pfaller, M.A. (2009). Medicial Microbiology (6th ed.). Philadelphia: Mosby Elsevier. |