Clostridium difficileClostridium difficile is a
Gram-positive, toxin-producing anaerobic bacterium
belonging to the family Clostridiaceae of the
Clostridiales. Though strictly oxygen-intolerant,
C. difficile is able to produce aerotolerant
endospores under unfavourable conditions that are capable of persisting
in an open environment for years. C. difficile is a
commensalist
species typically housed in the
colonic fecal flora of a fairly small
subset of the child population, with the number
of carriers decreasing as
children age (Kelly and LaMont, 1998). When it
exists in small numbers, this organism remains
non-pathogenic. However, when it does manage to colonize and
yield larger populations, its pathogenicity becomes the root
cause of a variety of colon
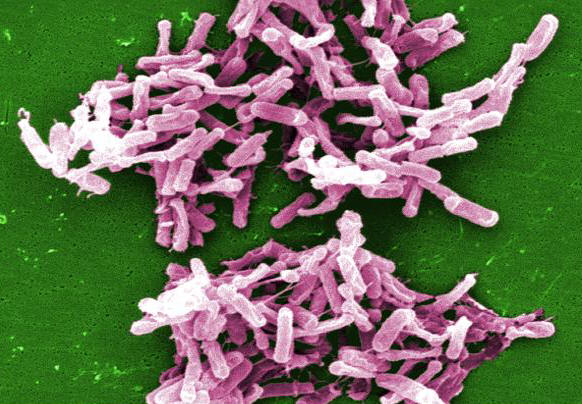
Figure 1. This micrograph depicts Clostridium difficile bacteria from a stool sample culture. Infection by C. difficile arises in an opportunistic manner; normal microflora in the gut attributes to the body’s defense against pathogenic domination and so, mass colonization of C. difficile usually occurs in individuals where typical colonic flora has been disrupted as a result of antibiotic use, individuals who are immunosuppressed (AIDS or cancer patients), or people taking so-called intestinal cleansers. C. difficile inherent pathogenicity lies in its ability to produce toxin. In particular, this pathogen produces two potent cytotoxins, namely, toxin A and toxin B, which ultimately lead to C. difficile-associated infection and disease (Mylonakis et al., 2001). Toxins A and toxin B are glucosyltransferases that target and inactivate the Rho family of GTPase enzmyes. Toxin A induces actin depolymerization by a mechanism correlated with a decrease in the ADP-ribosylation of the low molecular mass GTP-binding Rho proteins. Another toxin, binary toxin, has also been described, but its role in disease is not yet fully understood. Eventually, this leads to the deterioration of epithelial cell components and inevitably cell death. Additionally, both toxins induce a strong cellular inflammatory reaction and mass cytokine release, as well as activate the enteric nervous system, attracting neutrophils to the site (Jefferson et al., 1999). The overall outcome of toxin A and toxin B production is colonic mucosal injury and inflammation. C. difficile is transmitted from person to person by the fecal-oral route. Because the organism forms heat-resistant spores, it can remain in the hospital or nursing home environment for long periods of time. It can be cultured from almost any surface in the hospital. Once spores are ingested, they pass through the stomach unscathed because of their acid-resistance. They germinate into vegetative cells in the colon upon exposure to bile acids and multiply. C. difficile-associated infection is typically
restricted to the lower Pseudomembranous colitis caused by C. difficile is treated with specific antibiotics, such as vancomycin or metronidazole. To reduce complications, physicians often begin treatment based on clinical presentation before definitive results are available. Knowledge of the local epidemiology of intestinal flora of a particular institution can guide therapy. In addition, oral rehydration therapy is useful in retaining fluids during the duration of diarrhea. Interestingly, several disinfectants commonly used in hospitals may be ineffective against C. difficile spores, and may actually promote spore formation. However, disinfectants containing bleach are effective in killing the organisms and should constantly be used as a sanitizer. Laboratory culturing and Gram staining procedures are considered too unspecific to identify C. difficile in clinical situations due to its morphological similarity to other Clostridia species that make up the normal microflora of the colon. Typical diagnostic tests select for the presence of toxin A or toxin B production (Mylonakis et al., 2001). Tissue culture assay for specificity of toxin B cytotoxicity remains one of the most sensitive and exact tests used for diagnosis. Enzyme-linked immunosorbent assays (ELISAs) are also able to identify toxin A and/or toxin B in stool, which are rapid, specific, and most frequently used for clinical diagnosis of presumed C. difficile infections. References: Jefferson, K.K., Smith, M.F.
Jr., & Bodak, D.A. (1999). Roles of
intracellular calcium and NF-kappa B in the
Clostridium difficile toxin A-induced
up-regulation and secretion of IL-8 from human
monocytes. Journal of Immunology, 163:
5183-5191. |