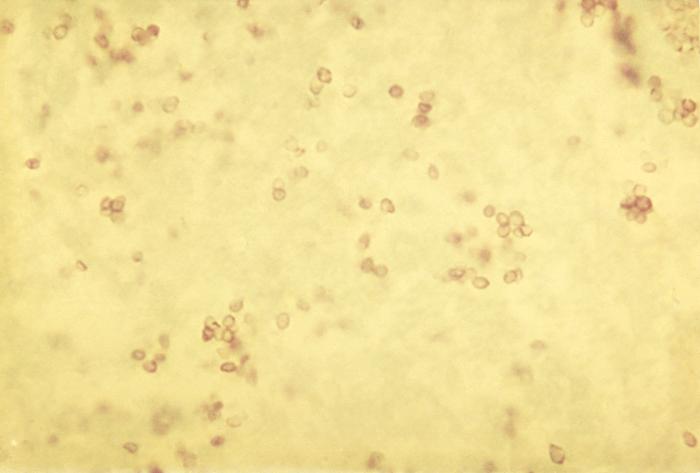
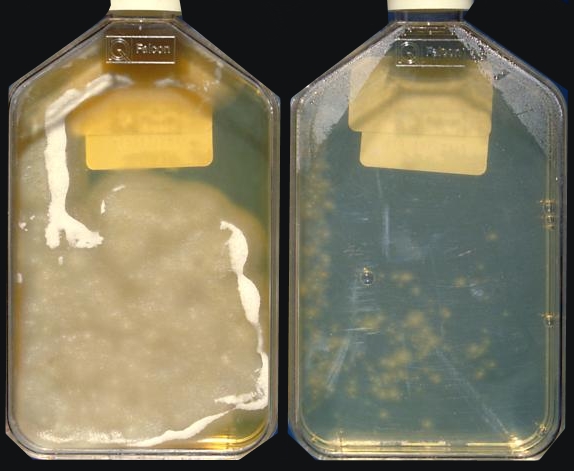
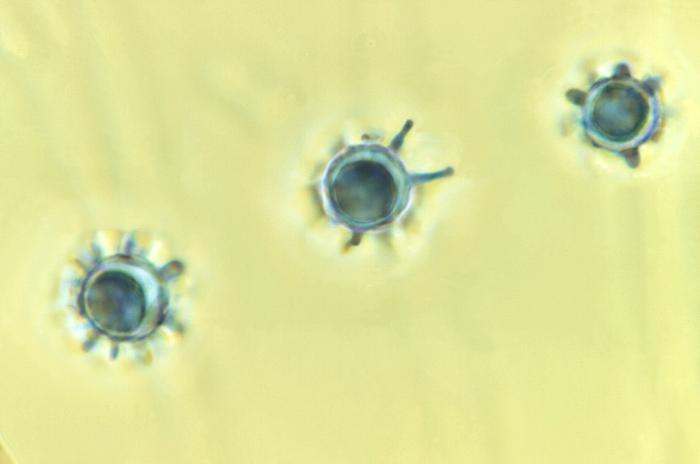
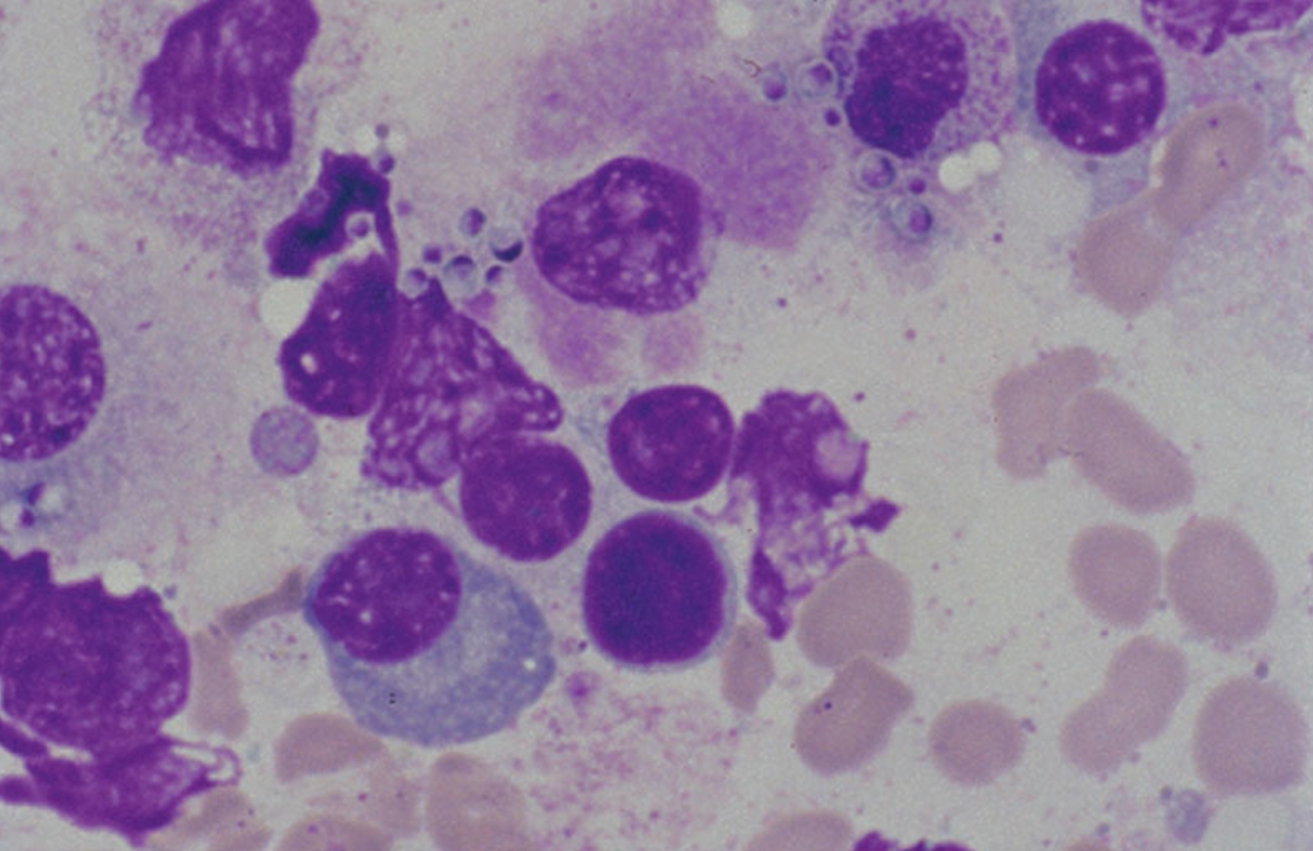
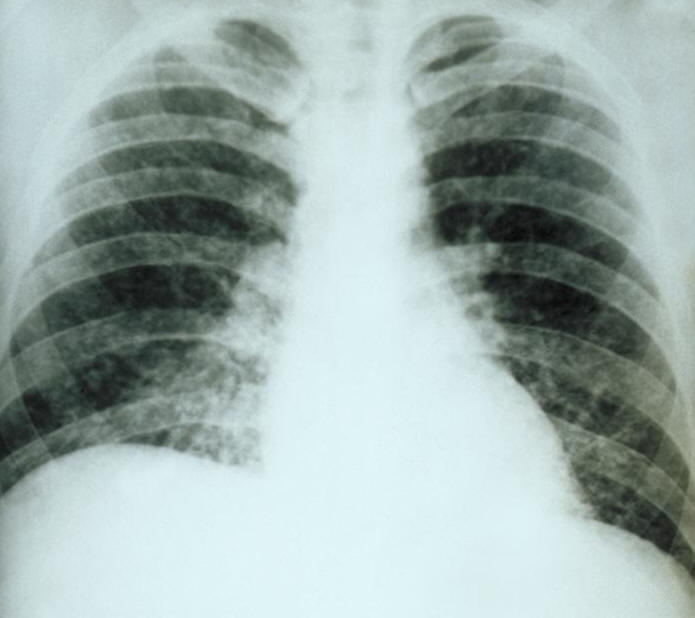
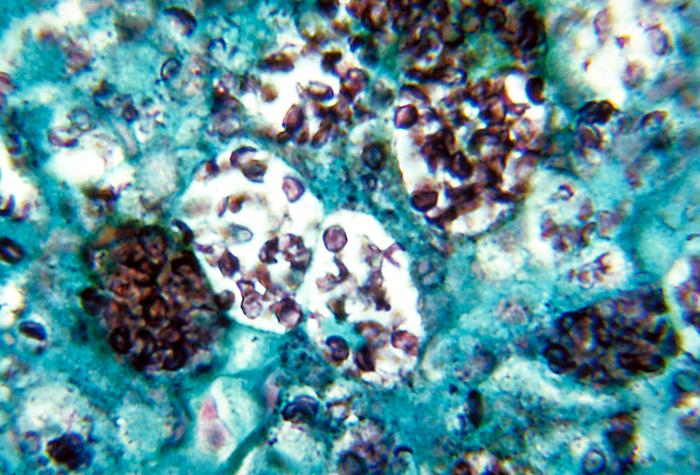
Histoplasma capsulatumOverview: Histoplasma capsulatum is a dimorphic fungus and the etiologic agent responsible for causing the infection known as histoplasmosis, which is manifested primarily as a lung disease in those parts of the world where this pathogen is endemic (Figure 1). At ambient temperatures, H. capsulatum remains in a saprophytic mycelial (mould) form and typically grows in soil and material contaminated with bat or bird droppings. Spores become airborne when contaminated soil is disturbed. When spores produced by this fungus are breathed in, the pathogen grows into the next stage of its life cycle – an encapsulated parasitic yeast. The disease is not transmitted from an infected person to someone else. Figure 1. Yeast forms of Histoplasma capsulatum in tissue. Life Cycle:
Dimorphism in H. capsulatum involves
three stages. In the first stage, induced by an
increase in temperature, respiration ceases and
the level of
cytochrome proteins decreases. During the
second stage of the mycelial-to-yeast
conversion, cysteine or other sulfhydryl-containing
compounds are required. Shunt pathways are
initiated that restore the appropriate
cytochrome levels, which supply the needed ATP
(energy molecule). Cysteine is also required for
the yeast form to grow. The final stage is
characterized by normal cytochrome levels and
respiration as the yeast grows and reproduces.
The conversion of terminal or intercalary hyphal
cells to a yeast form requires 3 to 14 days. In
tissue, H. capsulatum proliferates
within giant cells. It is also important to note
that this fungus shows thermal dimorphism, that
is, mould forms at 25°C and yeast forms at 37°C. Figure 2. The left bottle Histoplasma capsulatum culture growth is shown at eight weeks and the right bottle is shown at three weeks. These H. capsulatum cultures were grown from sediment of concentrated sputum on Sabhi agar with chloramphenicol, an antibiotic, and cycloheximide, an antibiotic fungicide having been added to the culture medium. Although the mycelial structure cannot be used for diagnostic purposes, it is an integral stage of the H. capsulatum life cycle, especially since it enables the species to survive and grow by spreading out hyphae in search of nutrition sources. The mycelial stage develops both macro- and micro-conidia, which aerosolize and are inhaled by unsuspecting people and other mammals (Figure 3). Hyphal fragments can also initiate infection, prompting a dramatic conversion of the mycelial form to budding yeasts. It is the macroconidia that exhibits finger-like projections from its surface, whereas, the microconidia are much smaller, round, and exhibit a smooth surface. Figure 3. This photomicrograph depicts the the presence of three Histoplasma capsulatum macroconidia, differentiated from the microconidia by the presence of projections emanating from their surface [1125 X]. Virulence: H. capsulatum yeast form is unique from other intracellular fungal pathogens in its ability to prevent the acidification of the phagolysosome following ingestion by alveolar macrophages (specialized phagocytes of the lungs), presumably limiting the antifungal effectiveness of this compartment. Residing in mammalian macrophages for a prolonged period of time causes respiratory and systemic disease. Furthermore, iron limitation is an important host antimicrobial defense, and iron acquisition is critical for microbial pathogenesis. H. capsulatum displays several iron acquisition mechanisms, including secreted γ-glutamyltransferase enzyme activity. Generally, iron is an indispensable micronutrient for nearly all living organisms, where it acts as a cofactor of many enzymes involved in manifold vital cellular and physiological functions. If iron is sequestered from its host, it severely compromises iron withholding-based host defense strategies and likely contributes to the increase in susceptibility to some fungal and bacterial infections. The enzyme γ-glutamyltransferase reduces iron by generating an efficient ferric reductant (Zarnowski et al., 2008). Clinical Infections: Symptoms of histoplasmosis vary greatly, but the disease primarily affect the lungs. Occasionally, other organs are affected. This form of the disease is called disseminated histoplasmosis, and it can be fatal if untreated. Skin lesions can be a manifestation of disseminated histoplasmosis, referring to the spread of the fungal pathogen, H. capsulatum, throughout the body including the skin, bone marrow, brain and other organs (Figure 4). Disseminated histoplasmosis is most common amongst immunosuppressed people, such as those with AIDS. 90% of infections are asymptomatic, or result in a mild influenza-like illness. Some infections, however, cause acute pulmonary histoplasmosis as manifested by high-grade fever, headache, a non-productive cough, chills, weakness, and pleuritic chest pain (Figure 5). Figure 4. Bone marrow stained with Giemsa showing the yeast phase of Histoplasma capsulatum. Figure 5. This chest film shows diffuse pulmonary infiltration due to acute pulmonary histoplasmosis caused by Histoplasma capsulatum. Identification: Histoplasmosis, identified from the yeast stage, can be diagnosed through histochemical and PCR diagnostics, while a key diagnostic factor is whether the patient has travelled or resides in an area with high bat or bird feces concentrations (Figure 6). If patients have been in the presence of these areas, then the diagnosis is made easier, but if this is not the case the diagnosis of H. capsulatum is very difficult. PCR has been deemed a legitimate tool that can reduce the diagnosis time when compared to traditional plating methods. Figure 6. Note the histopathologic changes seen in histoplasmosis due to Histoplasma capsulatum using methenamine silver stain. Note the presence of typical yeast cells, some of which are undergoing replication by budding. Histoplasmosis can be confined to the lungs, or become systemically disseminated, thereby, producing a fatal outcome. Treatment: Antifungal medications are used to treat severe cases of acute histoplasmosis and all cases of chronic and disseminated disease. Typical treatment of severe disease first involves treatment with amphotericin B, followed by oral itraconazole. Treatment with itraconazole will need to continue for at least a year in severe cases. In many milder cases, oral itraconazole or ketoconazole is sufficient. Asymptomatic disease is typically not treated. Past infection results in partial protection against ill effects if reinfected. Vaccination: Laboratory vaccines for this pathogen have focused on surface glycoproteins termed H and M proteins, cell wall proteins, and heat shock proteins. Immunity appears to be primarily through a T helper 1 cell-mediated immune pathway. The particular efficacy of a given vaccine candidate may depend on the rote of infection. For example, the H glycoprotein does not protect against systemic H. capsulatum infection but does protect against pulmonary infection (Pier et al., 2004). References: Pier, G.B., Lyczak, J.B., & Wetzler, L.M. (2004). Immunology, Infection, and Immunity. Washington: ASM Press. Maresca, B., & Kobayashi, G.S. (1989). Dimorphism in Histoplasma capsulatum: a Model for the Study of Cell Differentiation in Pathogenic Fungi. Microbiological Reviews, 53(2): 186-209. Zarnowski, R., Cooper, K.G., et al. (2008). Histoplasma capsulatum secreted γ-glutamyltransferase reduces iron by generating an efficient ferric reductant. Molecular Microbiology, 70(2): 352-368. |