Pneumocystis jiroveciOverview: Formerly known as Pneumocystis carinii, and classified as a protozoa, investigative tests upon this organism’s nucleic acid and biochemical composition has since placed it in the Kingdom of Fungi. Pneumocystis jiroveci is found in the lungs of mammals, where it resides in the alveoli without causing overt infection until the host's immune system becomes debilitated, causing the fungal infection Pneumocystis pneumonia or pneumocystosis. Thus, the organism causes disease in immunosuppressed individuals, including those with AIDS or undergoing chemotherapeutic treatments. P. jiroveci is specific to humans; it has not been shown to infect other animals, while other species of Pneumocystis that parasitize other animals have not been shown to infect humans.
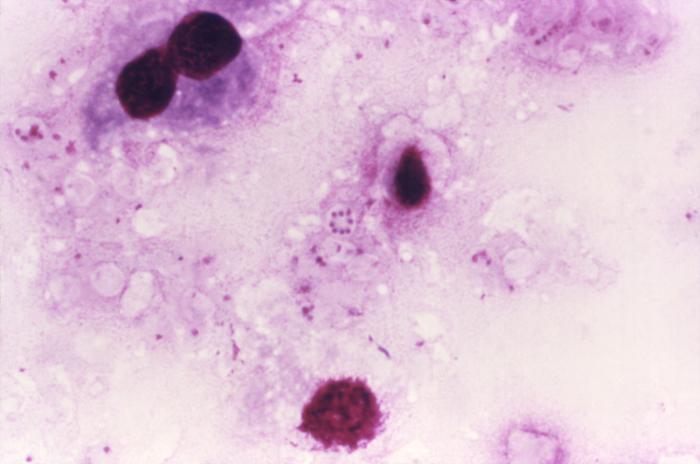
Figure 1. This photomicrograph reveals Pneumocystis jirovecii fungi, which were present in this Giemsa-stained impression smear of rat lung tissue. Note the round cyst in the very middle of this image containing eight immature haploid neuclei, as well as numbers of freed trophozoites [1000 X]. Biology: P. jiroveci is a non-motile unicellular eukaryote, traditionally described in two different forms. The small trophic forms 1 μm (micrometres) to 5 μm in size are pleomorphic and ameboid in shape, generally represent the majority of organisms identified in the lungs of animals with P. jiroveci pneumonia, and frequently exist as large clusters. The larger cyst forms 5 μm to 8 μm in diameter are the most readily recognized forms of the organism, although representing only a small percentage of organisms in the lungs of animals with P. jiroveci pneumonia. The smooth spherical cyst form is enclosed by a thick cell wall and may contain up to eight intracystic bodies (sporozoites). These intracystic bodies may represent precursors to tropic forms which may develop upon release by the cyst, although the life-cycle has not been established and is poorly understood. Life Cycle: In the asexual phase, trophic forms replicate by mitosis. In the sexual phase, haploid trophic forms conjugate and produce a zygote or sporocyte (early cyst) (Figure 2). The zygote undergoes meiosis and subsequent mitosis to produce eight haploid nuclei (late phase cyst). Spores exhibit different shapes (such as, spherical and elongated forms). It is postulated that elongation of the spores precedes release from the spore case. It is believed that the release occurs through a hole in the cell wall. After release, the empty spore case usually collapses, but retains some residual cytoplasm. A trophic stage, where the organisms probably multiply by binary fission is also recognized to exist.
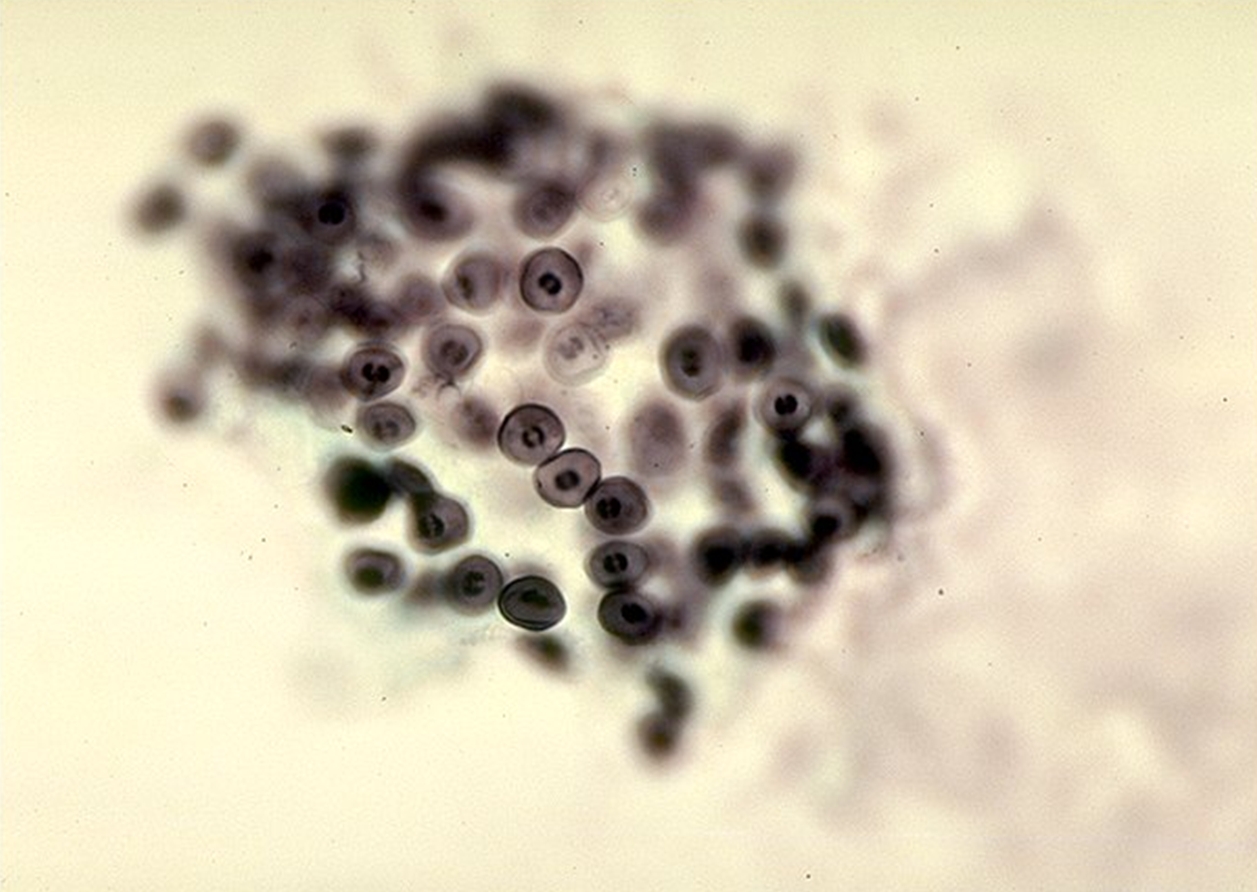
Figure 2. Pneumocystis jiroveci cysts. Virulence and Pathogenicity: The attachment of P. jiroveci to lung epithelial cells, upon inhalation of the trophozoite (replicating) form, appears to be a critical step in establishing disease in the susceptible host. Attachment to lung epithelial cells (type I pneumocytes) may be mediated in part by a variety of host molecules, including fibronectin, vitronectin, laminin, and mannose receptors. Type I pneumocytes (squamous alveolar cells) are responsible for gas exchange in the alveoli and cover a majority of the alveolar surface area (>95%). Binding may be facilitated by P. jiroveci extracellular matrix receptors. In the susceptible host, attachment of P. jiroveci to alveolar epithelial cells is associated with proliferation of the organism and impaired lung cell replication, perhaps through secretion of proteolytic enzymes such as chymase or reactive oxygen species (Patel & Koziel, 2004). Although specific virulence
factors are poorly defined, P. jiroveci
can induce disease in the susceptible host
through a number of strategies. P. jiroveci
induces selective alterations in expression and
biophysical activity of lung surfactants, and
may also have effects on non-epithelial lung
cells such as alveolar macrophages and lung
monocytes by down-regulation of important cell
transcription factors. By coating itself in
host-derived glycoprotein molecules from the
lung microenvironment such as surfactant
protein-A and a soluble form of the macrophage
mannose receptor, P. jiroveci may delay
recognition and destruction by the host immune
system, to enable proliferation and survival
(Patel & Koziel, 2004). P. jiroveci replicates extracellularly and impairs oxygen diffusion. Inflammation induced by this pathogen causes host cell damage, resulting in cell lysis and rupture. Damage to the lung basement membrane generates a characteristic foamy exudate and interstitial leukemic infiltration in the alveoli, thereby reducing alveolar capillary permeability. Granulomatous inflammation in Pneumocystis pneumonia is rare but may be seen in up to 5% of lung biopsies from human immunodeficiency virus (HIV)-infected patients. Identification:
Prior to the mid-1990s, the only way of
detecting the presence of a Pneumocystis
infection was through the visualization of the
fungal microbe from stained respiratory tissues
using Giemsa and Gomori-Grocott techniques,
staining sputum, branchoalveolar fluid or lung
tissue (Thomas et al., 2004). When
staining, the firm-walled cyst form is most
visible, but the flexible-walled trophic form
outnumbers the cyst form 1:10 and the trophic
form is difficult to visualize (Forbes et al.,
2003). Therefore, staining is not the most
convenient way of detecting infection.
Polymerase chain reaction (PCR) is now used to
identify the presence of the organism in medical
specimens, which has increased the sensitivity
of detecting the microbe. Treatment: Antifungal medications do not kill P. jiroveci. The antibiotic Trimethoprim-sulfamethoxazole is the primary treatment for all severity of pneumocystis pneumonia. Trimethoprim is an inhibitor of dihydrofolate reductase and sulfamethoxazole is an inhibitor of dihydropteroate synthase. The microbe stays within hosts for weeks after therapy has begun. It is of extreme importance that patients with AIDS take the preventative methods to avoid pneumocystis pneumonia because without treatment, over 85% of people with HIV develop the infection (Stringer et al., 2002). References: Patel, N., & Koziel, H. (2004). Pneumocystis jiroveci Pneumonia in Adult Patients with AIDS: Treatment Strategies and Emerging Challenges to Antimicrobial Therapy. Treatments in Respiratory Medicine, 3(6): 381-397. Stringer, J.R., Beard, C.B., et al. (2002). A New Name (Pneumocystis jiroveci) for Pneumocystis from Humans. Emerging Infectious Diseases, 8: 891-896. |