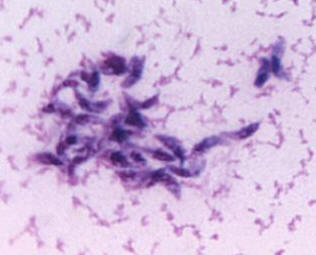
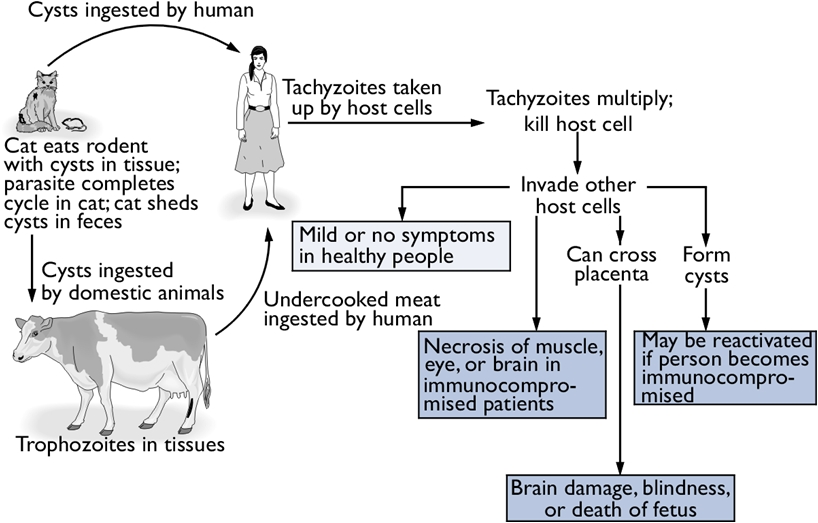
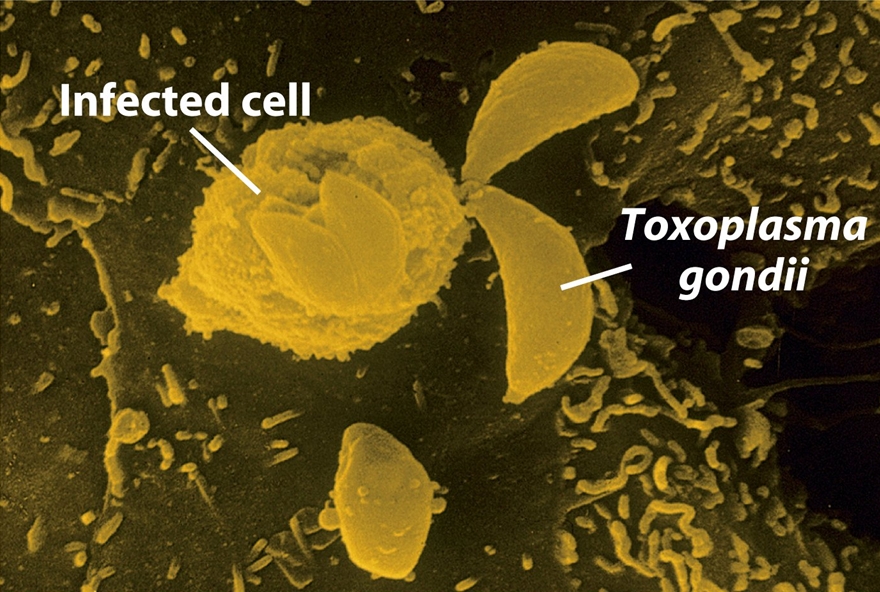
Toxoplasma gondiiOverview: Toxoplasma gondii is a single-celled, intracellular protozoan that causes the parasitic diseases known has Toxoplasmosis (Figure 1). T. gondii has a very broad host specificity, such that this parasite can infect and become encysted in the tissues of virtually any warm-blooded animal. The definitive host is the cat (both domestic and wild) where parasite sexual development occurs in the small intestine. Other mammals, including humans, can become infected when they eat the eggs deposited in cat feces or eat undercooked meat from an infected animal (Figure 2). T. gondii has a tropism for neural tissue and normally remains encysted in the central nervous system. Infection is very common, and it is estimated that over a third of the world's population has been exposed. Although the infection is generally asymptomatic, there are two groups of people who are at significant risk for disease: immunocompromised individuals and pregnant women. Figure 1. Toxoplasma gondii in mouse ascitic fluid. Figure 2. Progression of a Toxoplasma gondii infection and consequences for the human host. Click to enlarge. Life Cycle: The family Felidae is the definitive host, and sexual stages of the parasite are found in the epithelial cells of the gut, and oocysts are shed in the feces. In the intermediate host (any mammal or bird), the parasite undergoes asexual division in one of two stages. The tachyzoite is rapidly dividing and can infect any nucleated cell, hence the wide range of hosts. In the absence of a T-cell response, unrestricted growth of tachyzoite will kill the host. Normally, in the presence of a competent immune system, the tachyzoite stage is cleared from the tissues while the more slowly dividing form of the parasite, persists within pseudocytes. An active T-cell response to the parasite is required to prevent reemergence of the virulent tachyzoites from pseudocytes. There are three routes of transmission: (1) horizontal transmission via oocysts; (2) horizontal transmission via tissue cysts (rodent to cat or pig to human), and (3) vertical transmission (congenital infection) via tachyzoites (Figure 2). T. gondii are motile species. Their motility is powered by an actomyosin system that allows the tachyzoite to disperse throughout tissues and penetrate host cells via upright twirling, helical rotation, and circular gliding. The latter motion induces the secretion of calcium ions from the parasite, which is a unique feature that helps parasitologist identify the organism. However, the main method used to identify T. gondii is by identifying the tachyzoite form using a blood sample from the suspected host using the polymerase chain reaction technique. Virulence and Pathogenicity: T. gondii infects not only macrophages but also many other cell types in an uptake process actively controlled by this protozoan (Figure 3). Unlike the protozoan Leishmania major, Toxoplasma parasites avoid destruction by preventing fusion of the phagosome with the lysosome and live permanently in a unique parasitophorous vacuole, a specialized structure within the cytoplasm that is entirely segregated from the endocytic system of the host cell. The parasite thereby avoids exposure to lysosomal enzymes and an acidic environment. The parasite replicates to large numbers until the infected cell bursts, releasing the pathogen. This organism is also able to cause infection by modulating the host immune response. It does this using the parasitic heat shock protein TgHSP70, which induces maturation in host dendritic cells; when the dentritic cells mature, their phagocytic activity decrease. Figure 3. A host cell infected by Toxoplasma gondii parasites. Click to enlarge. Immune Response: Infection via the gastrointestinal route elicits immunoglobulin A (IgA) responses that can be diagnostic of infection. Both humoral and cellular responses are part of the normal reaction to infection, but protective immunity is best correlated with cell-mediated immunity. Lytic CD8+ T cells are likely important effectors of immunity, and cytokines produced by T cells, such as interferon-γ and tumor necrosis factor-α, appear critical for mediating immunity. Clinical Infections: T. gondii can produce acute or chronic infection. These usually do not provoke symptoms in immunocompetent hosts. During acute toxoplasmosis, symptoms are often influenza-like: swollen lymph nodes, or muscle aches and pains that last for a month or more. Rarely, a patient with a fully functioning immune system may develop eye damage or nasal lesions from toxoplasmosis. Those who are immunocompromised (people with AIDS or cancer) may develop severe toxoplasmosis. This can cause damage to the brain (encephalitis) or the eyes (necrotizing retinochoroiditis). Infants infected via placental transmission may be born with either of these problems, or with nasal malformations. Infections of pregnant women is an important public health issue, as a primary infection during pregnancy can lead to infection of the fetus, causing abortion or congenital disease, including mental retardation and blindness. Swollen lymph nodes are more commonly found in the neck followed by axillae and then groin. Swelling may occur at different times after the initial infection, persist, and/or recur for various times independently of antiparasitic treatment. It is usually found at single sites in adults, but in children multiple sites may be more common. Enlarged lymph nodes will resolve within one to two months in 60% of patients. Most patients who become infected with T. gondii and develop toxoplasmosis do not know it. In most immunocompetent patients, the infection enters a latent phase, during which only bradyzoites are present, forming cysts in nervous and muscle tissue. Most infants who are infected while in the womb have no symptoms at birth but may develop symptoms later in life. Treatment: For acute toxoplasmosis, the following medicines can be administered, namely, pyrimethamine, sulfadiazine, clindamycin, cotrimoxazole, and/or spiramycin. In people with latent toxoplasmosis, the cysts are immune to these treatments, as the antibiotics do not reach the bradyzoites in sufficient concentration. Medications that are prescribed for latent toxoplasmosis are: atovaquone and clindamycin, an antibiotic which, in combination with atovaquone, seems to optimally kill cysts in mice. Vaccination: As with most parasites, vaccines for toxoplasmosis are all experimental. Purified and recombinant proteins, such as surface antigen 1 (SAG1), granule antigen 1 (GRA1), GRA7, and rhoptry protein 2 (ROP2) - rhoptries are apical organelles that release their contents during the invasion process - elicit partial or full protection in animals. DNA vaccines directed against these antigens has also shown efficacy in animals studies. References: Pier, G.B., Lyczak, J.B., & Wetzler, L.M. (2004). Immunology, Infection, and Immunity. Washington: ASM Press. |