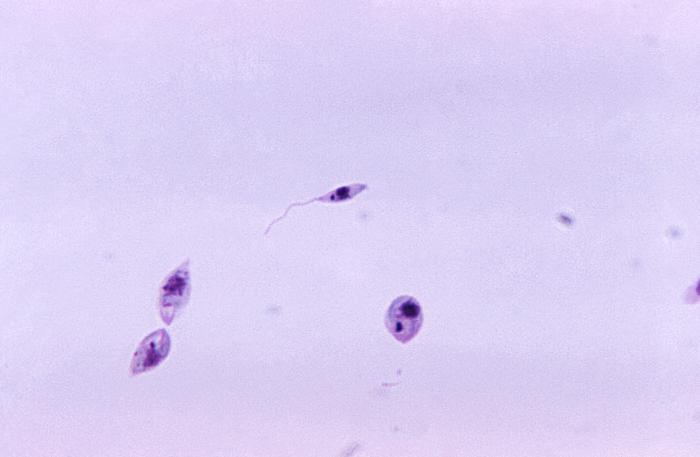
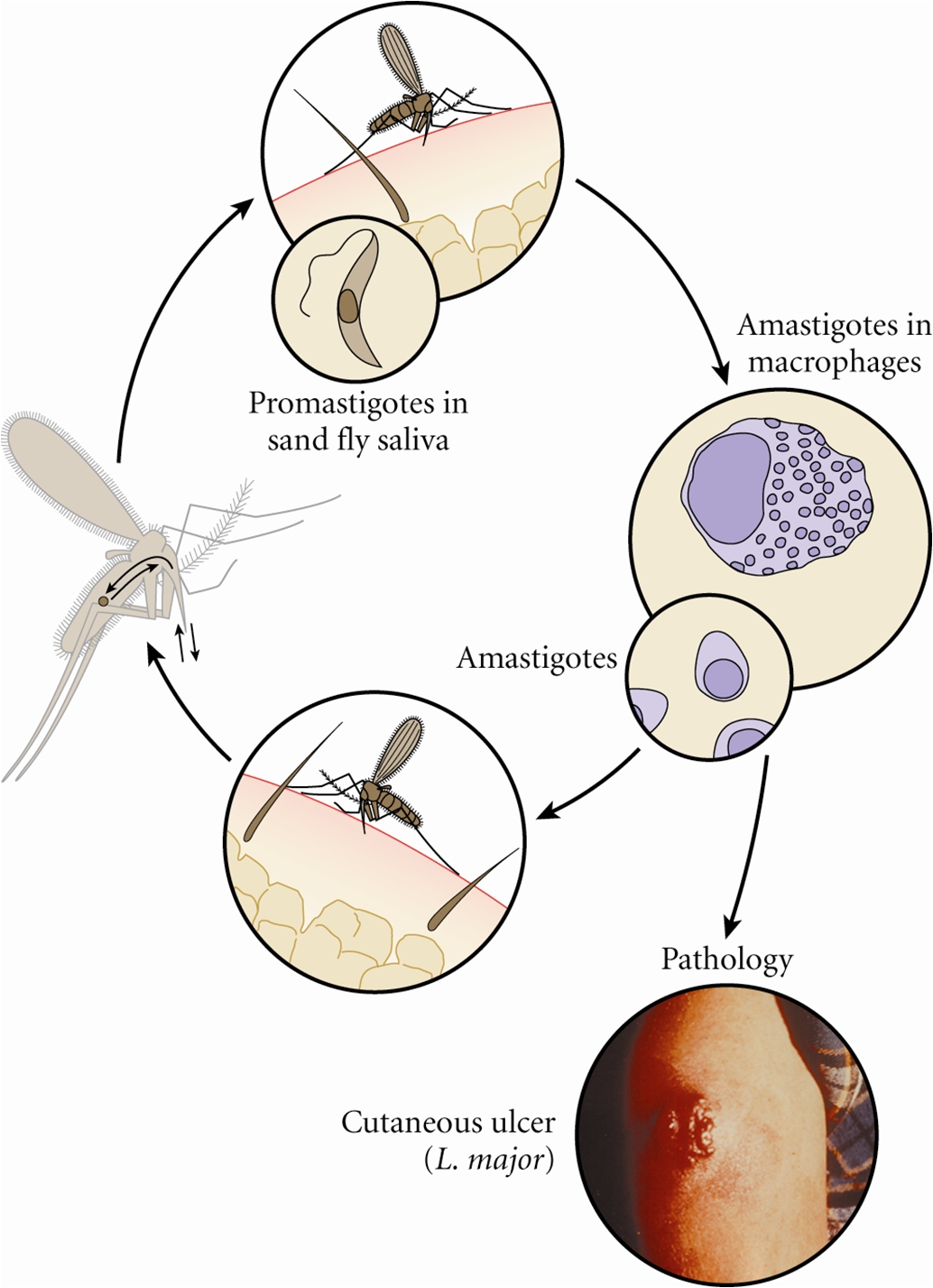
Leishmania majorLeishmania major is one of many protozoan parasites of the genus Leishmania that causes Leishmaniasis, a cutaneous and visceral intracellular protozoal infection. In total there are at least 23 species belonging to the genus Leishmania. These pathogens exist in two distinct parasitic stages, namely, the promastigote stage and amastigote stage. The promastigote is the infective-stage and is transmitted by the bite of a sandfly (Figure 1). The promastigote stage differs from the amastigote stage as it possesses flagella, in addition to glycoproteins and mannose receptors on its membrane. These are used for binding and attraction of the macrophages in the host. After the promastigote has been incorporated into a macrophage, it transforms into an amastigote, a semi-spherical organism that lives and multiplies via binary fission within endolysosomal vacuoles of the infected macrophage (Figure 2). There is no surface coat present on amastigotes and their membrane is therefore only composed of a single polysaccharide component.
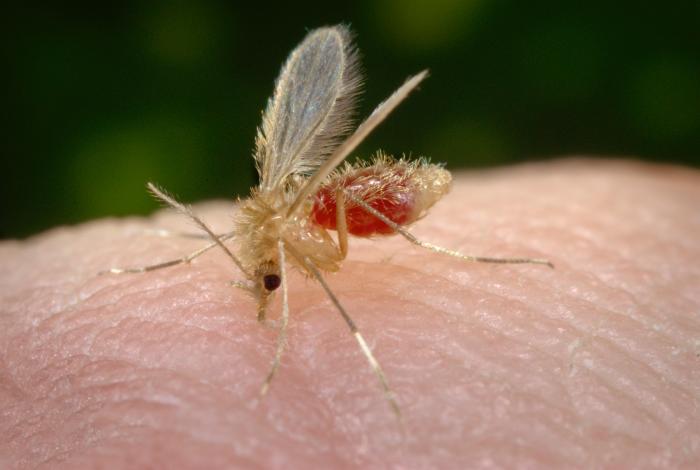
Figure 1. This micrograph reveals the presence of a number of Leishmania major promastigotes. Figure 2. Life cycle of Leishmania species. Click to enlarge. L. major is found in the Eastern hemisphere, specifically North Africa, the Middle East, West India and Sudan, while other species are dominant elsewhere. There are also different genera of sandflies that carry each species of Leishmania. L. major is carried by the female Phlebotomine sandfly (Figure 3). The sandfly can only pass the parasite onto humans or other mammals if the fly itself is infected. To become infected, the sandfly must feed on an already infected host. The parasite will be taken up by the fly in the amastigote form, develop into promastigotes within the insect's gut, migrate to the insect's salivary glands, and then deposited on the skin of another host when the sandfly takes its next blood meal (Figure 2). Promastigotes that are inoculated into the mammalian host following an infective sandfly bite activate serum complement and become opsonized with C3b protein. This coating of C3b triggers the uptake of the promastigotes into the macrophage without triggering the cell's respiratory burst. The phagosome containing the parasite fuses with the lysosome to form a late endolysosomal compartment. To survive within the hostile environment, the parasite produces antioxidant enzymes and inhibitors of lysosomal enzymes. Within the endolysosomal, the promastigote undergoes morphological transformation into its amastigote form. After several rounds of replication, the infected macrophage eventually ruptures, releasing the amastigotes. Figure 3. This photograph depicts a Phlebotomus papatasi sandfly, which had landed atop the skin surface of the photographer, who’d volunteered himself as host for this specimen’s blood meal. The sandflies are members of the Dipteran family, Psychodidae, and the subfamily Phlebotominae. This specimen was still in the process of ingesting its bloodmeal, which is visible through its distended transparent abdomen. Sandflies such as this P. papatasi, are responsible for the spread of the vector-borne parasitic disease Leishmaniasis, which is caused by the obligate intracellular protozoa of the genus Leishmania. Clinical Infections: Leishmaniasis is caused by a number of species of protozoan parasite parasite of the genus Leishmania. The disease can range from a local skin infection that usually resolves, called cutaneous leishmaniasis, to a mucocutaneous spread of infection, to a systemic infection involving parasites inside macrophages throughout the body, called visceral leishmaniasis. Cutaneous leishmaniasis is characterized by course ulcers to the skin. The ulcers themselves have distinguishing features, such as raised edges, with a central crater resembling a volcano (Figure 2). The ulcer may take a few weeks to months following the initial sandfly bite to manifest itself. Diagnosis of the Leishmania infection can be done by either observation or microscopic investigation. Observation methods look at the skin ulcers that do not heal. Microscopic methods culture the parasite taken directly from the sore. For the cutaneous infection caused by L. major, there is immunity to reinfection by the parasite. This means that subsequent bites by infected sandflies will not lead to the development of new ulcers. Cutaneous leishmaniasis is probably the only major human parasitic infection for which there appears to be immunity to reinfection. As with many other intracellular infections, cell-mediated immunity seems to be the most important mechanism of resolving the infection, with little if any role for antibodies (Pier et al., 2004). Leishmania antigens are presented to the host's T cells on MHC class II molecules, a feature thought to be due to the unique endosomal location of the intracellular parasite. Leishmaniasis has been intensively studied in murine models, which have demonstrated that CD4+ T helper type I (TH1) effector lymphocytes are the most critical components of acquired, protective cell-mediated immunity. These T cells produce cytokines that are necessary for activation of macrophages, which in turn destroy intracellular parasites by producing nitric oxide and reactive oxygen intermediates (Pier et al., 2004). Vaccines: Vaccine candidates include killed protozoal cells, usually mixed with an adjuvant; genetically modified, live, attenuated cells; DNA vaccines encoding recombinant proteins such as cysteine proteinases; and purified proteins. At present the development of an effective vaccine is limited by incomplete knowledge about which antigens are protective and the long-term safety of the various vaccine candidates under consideration and uncertainty about whether the use of drugs may be more feasible than vaccination for disease control (Pier et al., 2004). References: Pier, G.B., Lyczak, J.B., & Wetzler, L.M. (2004). Immunology, Infection, and Immunity. Washington: ASM Press. |