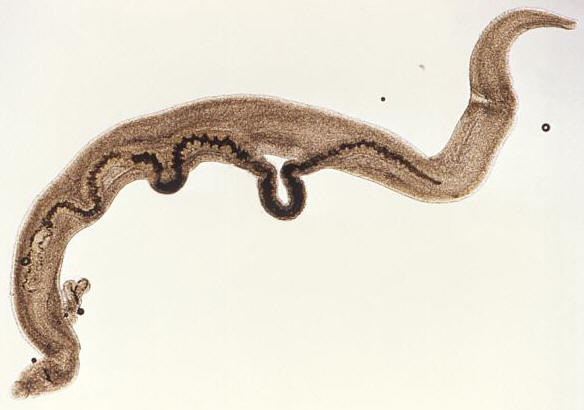
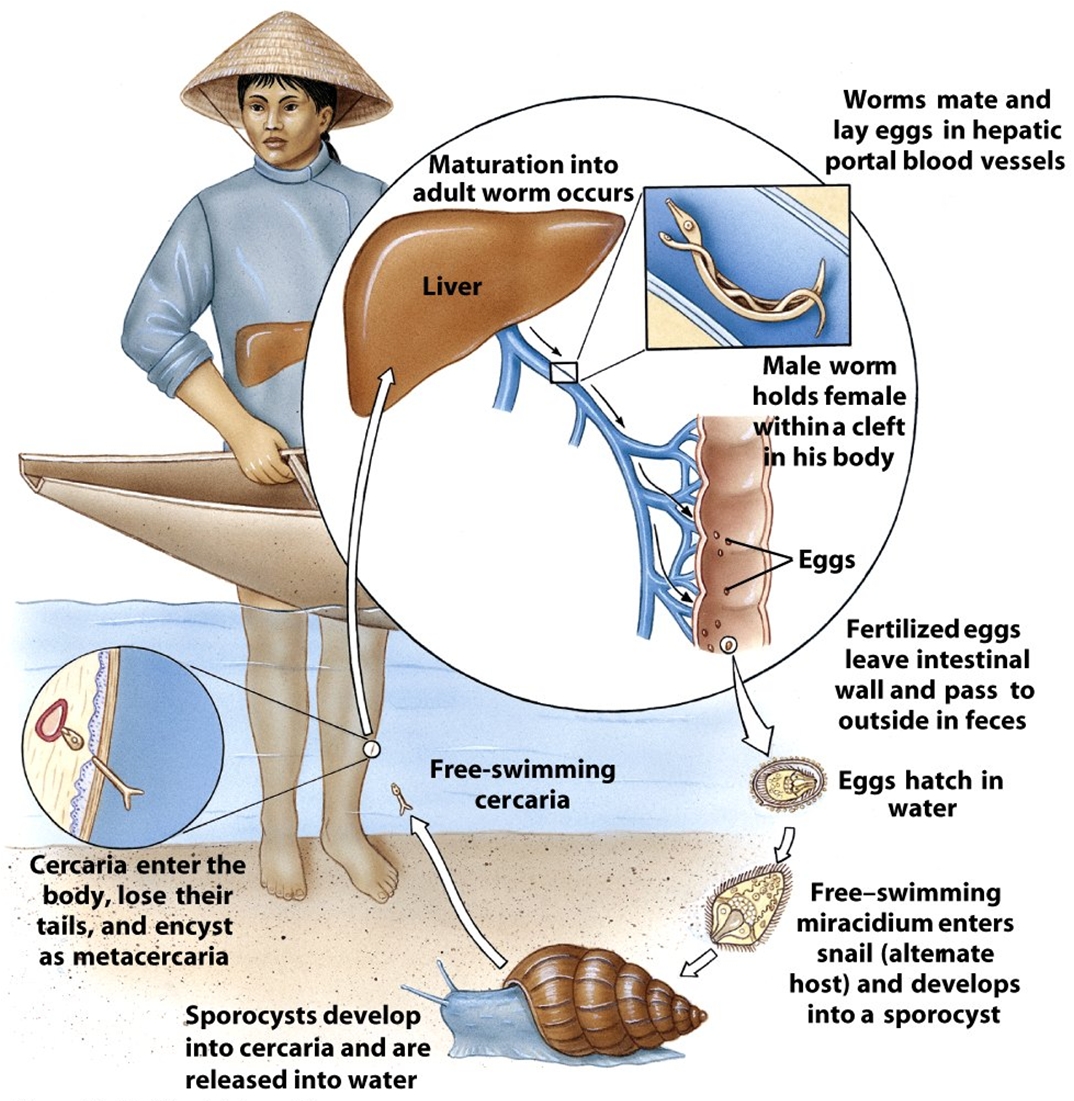
Schistosoma mansoniOverview: Schistosoma mansoni is one of the many trematode parasites of the genus Schistosoma that causes Schistosomiasis, a blood fluke infection transmitted by a freshwater snail (Figure 1). This multicellular pathogenic worm typically resides in the extracellular environment of human blood erythrocytes, tissue, and the intestinal tract. The lifecycle of Schistosomes includes two hosts: a definitive host such as a human, where the parasite undergoes sexual reproduction, and a single intermediate snail host, where there are a number of asexual reproductive stages. Human contraction of the parasite occurs during bathing or wading in fresh water inhabited by infected snails. Unlike most other trematode species, the adult stage of S. mansoni is characterized by two distinct sexes, as opposed to being hermaphroditic. The male S. mansoni species is light in colour and roughly one centimeter in length, while the female species is darker in colour, longer in length and thinner in width, allowing the females to reside within the male body and reproduce. Females can release thousands of eggs each day. The lifespan of adult worms can last a decade or longer, feeding on blood from the hepatic and mesenteric veins. Figure 1. This magnified view reveals four Schistosoma mansoni trematodes. At the far left is a pair of worms, where the thinner female is cradled inside the thicker male worm's gynecophoral canal. In the center is a single thin female worm, and at far right is the more robust, thicker male S. mansoni. Life Cycle: Humans become infected with schistosome parasites by standing or swimming in fresh water infested with schistosome-infected snails. Infective schistosome larvae called cercariae emerge from infected snails and actively penetrate human skin. In the skin, the larvae of S. mansoni transform into schistosomules, which migrate to the lungs and the portal vein of the liver, were the developing male and female schistosomes pair, settle in the mesenteric veins, and mature into adult worms. In these blood vessels, the female adult worm lives tightly ensconced within a longitudinal canal of the male worm and continuously produces eggs (Figure 2). Although most eggs are passed out in the feces, others are carried retrograde by the portal venous system to the liver, where they become trapped in the portal triad. These eggs hatch in fresh water to release miracidia that infect snails and develop into infective larvae. The stages in the snail include two generations of sporocysts and the production of cercariae. The photo in Figure 3 shows a patient with an enlarged spleen and liver due to S. mansoni infection. Figure 2. A pair of mating
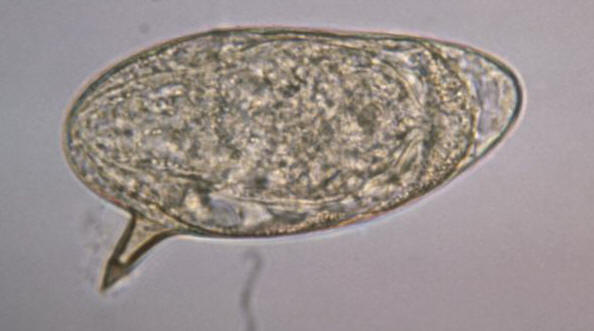
Schistosoma mansoni trematodes. Note that
the thinner female is cradled inside the thicker
male worm's gynecophoral canal. While inside
their human hosts, the adult works reside in the
mesenteric venules in various locations. Figure 3. Life cycle of S. mansoni. Click to enlarge. Virulence and Pathogenicity: The adult worms live for a decade of more in abdominal veins or blood vessels of the bladders, producing eggs that, when not expelled, lodge in the bowel wall over the long and fecund lifetime of the adult worms. S. mansoni eggs are considerably immunogenic and possess a distinctive spine (Figure 4). Glycoproteins present on the schistosome egg surface are the most immunoreactive components of the egg surface. Oligosaccharides derived from these egg glycoproteins contain the Lewisx trisaccharide, which stimulates large quantities of host T-helper 2 cell-driven interleukin-10 (IL-10) cytokine production. Figure 4. This micrograph depicts an egg from the parasite Schistosoma mansoni, and reveals the egg’s characteristic lateral spine. S. mansoni eggs are large, measuring 114 µm to 180 µm in length, and have a characteristic shape, with a prominent lateral spine near their posterior end. The anterior end is tapered, as well as slightly curved. The T-cell-mediated host reaction to these eggs results in the formation of granulomas, which in turn produce the pathological consequences of hepatic fibrosis (liver scarring) and portal hypertension (high blood pressure in the liver vein). These granulomas consist of T cells, B cells, macrophages, fibroblasts, and a large number of eosinophils, and though largely mediated by TH2 cells, T helper 1- (TH1)-type responses can contribute to the tissue destruction. Early responses to the egg antigens are of both the TH1 type and TH2 type, with a subsequent shift to a long-lasting TH2 response. Recall that a TH2 response is associated with the production of interleukin-10 (IL-10); IL-10 down-regulates interleukin-5 (IL-5) and interferon-γ, significantly reducing liver damage associated with the granulomas. IL-10 also reduces the expression of the costimulatory molecules CD80 and CD86 on the local macrophages. Presentation of egg antigens in the absence of these CD28 costimulatory molecules leads to T-cell anergy, thus reducing the intensity of the inflammatory response generated against eggs antigens. Similarly, continous activation of TH2 cells yields considerably high amounts of interleukin-4 (IL-4). IL-4 activates B cells to elicit a high level of immunoglobulin E (IgE) antibody during human infection. Moreover, S. mansoni parasites are resistant to complement-mediated lysis of their host. In fact, they are protected from the lytic C5b-9 complex by preventing the assembly of the complement cascade-amplifying enzyme C3 convertase by acquiring a molecule called decay-accelerating factor directly from human erythrocytes, which blocks assembly and accelerates the decay of C3 convertase, and in turn, inhibiting complement-dependent damage. Clinical Infections: Symptoms of schistosomiasis include internal bleeding, organ damage, severe anemia, and chronic diarrhea. However, it is possible not experience any signs or symptoms until a high accumulation of lodged eggs are produced. Research has shown that a large percentage of S. mansoni genes code for protease enzymes, which break down proteins and are therefore useful for penetration of the skin and other tissues. S. mansoni are also able detect light, temperature, and certain chemicals to aid in navigation within waters and host organism; thus, they have a developed neurosensory systems. In addition, there is little evidence for protective immunity to this parasite, and therefore it is possible to be become reinfected by the larvae. Studies have reported that adults are less likely than children to become reinfected because of lower susceptibility to infection, which could be due to selective immunity. In other words, the adult worms could induce immune responses that would protect against the infective larvae, but would not affect the already established adult worms, allowing the adult forms to survive by redirecting host immune response to a different life stage. Treatment: Schistosomiasis is readily treated using a single oral dose of the drug praziquantel. Antimony has been used in the past to treat the disease. In low doses, this toxic metalloid bonds to sulfur atoms in enzymes used by the parasite and kills it without harming the host. This form of treatment, however, is not universally recognized; praziquantel, on the other hand, is most often used. As with other major parasitic diseases, there is ongoing and extensive research into developing a schistosomiasis vaccine that will prevent the parasite from completing its life cycle in humans. References: Pier, G.B., Lyczak, J.B., & Wetzler, L.M. (2004). Immunology, Infection, and Immunity. Washington: ASM Press. |
Antimony: A brittle, lustrous, white metallic element occurring in nature free or combined, used chiefly in alloys and in compounds in medicine. Chemical symbol is Sb (below).
|