Hepatitis E VirusOverview: Hepatitis E virus (HEV) is the leading cause of acute viral hepatitis in the world, and is most commonly found in Africa, Central Asia, and Mexico. The virus is credited with half of all outbreaks of acute hepatitis in children and adults, specially in areas where it is endemic. Worldwide, infection caused by this pathogen is more widespread than infections caused by hepatitis A. Researchers suspect that 20% of the world's population has been infected with HEV, where it is more common in people who are between the ages of 15 and 40 years. In young children, infections caused by HEV are usually non-symptomatic, but in adults, cases are often more severe.
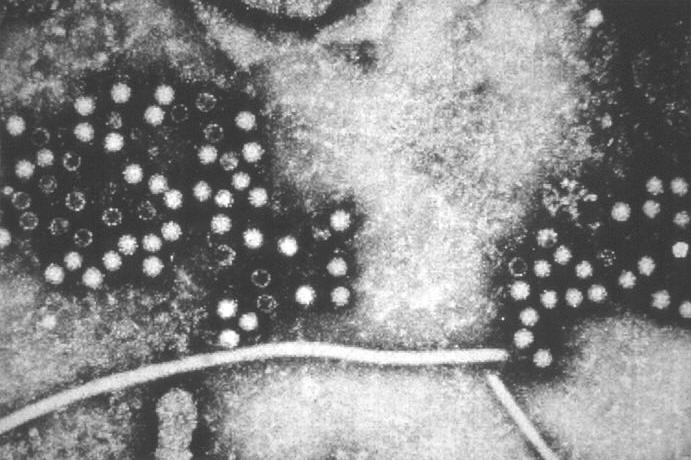
Figure 1. This electron micrograph depicts hepatitis-E viruses (HEV), provisionally classified as members of the Caliciviridae family. Unlike hepatitis A, HEV is transmitted through food or water contaminated by feces of infected people, as opposed to being spread by close contact from person to person. Like hepatitis A, however, HEV infection never becomes a chronic illness or long-term. In adults, hepatitis E is more serious than hepatitis A, with mortality rates of 1 to 2 percent. In contrast, the rate of adult mortality caused by hepatitis A is less than 0.4%. Moreover, symptoms associated with hepatitis E are similar to other types of viral hepatitis, such as malaise, abdominal pain, jaundice, and fever. The acute phase of the illness may last less than two weeks. Though the symptoms are non-existent in children and mild in most adults, infection during pregnancy can be fatal. As with hepatitis A, there are no treatments for HEV infection. Similarly, no vaccines have been developed to prevent HEV infection. Identification: In 1955, an epidemic of acute hepatitis hit New Delhi, India, after untreated sewage had contaminated drinking water in the city, affecting approximately 29000 people. At that time, health officials assumed that it was an outbreak epidemic of hepatitis A. In the early 1990s, scientists tested blood samples from some patients affected by New Delhi epidemic, which had been extracted and then stored. They found a new infectious viral agent that they called intestinal (enteric) hepatitis (associated with the gut or intestines), which was neither hepatitis A or hepatitis B. According to Centers for Disease Control and Prevention (CDC), between 15 and 25% of pregnant women infected with hepatitis E die. In a retrospective study in the early 1990s, scientists were able to identify molecular components of this pathogen that differentiated from other liver viruses and henceforth this virus became known as viral hepatitis E. Choosing the letter "E" also made sense alphabetically, because they scientists had already identified and named other hepatitis viruses, namely, A, B, C and D prior to HEV's discovery. Some scientists also interpret the E for enteric hepatitis. Like hepatitis C, HEV is a non-enveloped, single-stranded, positive-sense RNA virus that has not been conclusively identified as a member of any known virus family, although it does share some features with the Calicivirus family. However, biochemical analysis shows that the pathogen's viral RNA is more closely related to the Rubella virus, a Togavirus, than to members belonging to the Caliciviridae family. The virus is also spherical in shape and is approximately 32 nm (nanometers) to 34 nm in diameter (Figure 1). Pathogenicity: When the virus first encounters its target cell, its genetic material is deposited into the host cell's cytoplasm, where it is subsequently transcribed and translated into viral proteins, thereby producing progeny viruses. Most of the replication occurs in the liver, and virus particles are present in bile and feces of infected people for the first week of infection. The incubation period of the virus in humans can range from three to nine weeks. Symptoms: Patients with symptoms show some typical acute hepatitis, such as nausea, anorexia, fever, upper abdominal pain, dark urine, and jaundice - yellowing of the skin and whites of the eyes. However, the disease can also be silent, without symptoms, which is common in children. In two studies with volunteers, the elevation of enzymes, which occurs when the liver is irritated and hepatocytes are either damaged or dead, occurred four to five weeks after oral ingestion and persisted for 20 to 90 days. During acute infection, infected individuals may see a rise bilirubin (bile pigments) in the blood and urine, and a slight increase in alkaline phosphatase, an enzyme of the bile ducts. In the study, the elimination of the virus in human faeces, occurred about four weeks after ingestion virus and persisted for about two weeks. Only 1 to 2% of pregnant patients infected with HEV experienced symptoms of severe liver disease or fatal. The disease usually goes away within a period of two weeks. It is unclear whether infection with this virus confers lifelong immunity against future infections, as in the case of infected with hepatitis A. It is suggested that hepatitis E may play a role in causing the potentially fatal hepatitis in patients infected with hepatitis C. However, to date, there is no detailed evidence that HEV infection increases the severity of other virus-caused liver diseases in adults or children who already have chronic viral hepatitis. Epidemiology and Transmission: Historically, researchers had thought that HEV infection was endemic to India, Central Asia, parts of Africa and Mexico. This, however, is a false notion and highly outdated. The largest outbreak of reported hepatitis E occurred in Xinjing, China, between 1986 and 1988 with more than 119000 documented cases. In India, 70% of HEV infections occur in children. In fact, studies have shown that 70% of HEV infections in children were caused by contaminated drinking water and 20% due to contaminated food. These studies suggest that 9.5% resulted from contact in the home, which is inconsistent with the report of the CDC claiming the disease is not transmitted from person to person or household contact. Some studies have also reported a few cases of vertical transmission (from mother to child during birth) if the mother had hepatitis E during the third trimester of pregnancy. Hepatitis E virus is also found in breast milk, but there are no known documented cases of HEV transmission to infants through breast milk. Among blood donors worldwide, the CDC has reported that from 11 to 25% of developing countries prove to be positive for HEV antibodies. Scientists cannot confirm that HEV infection confers lifelong immunity against infection. Therefore, the molecular medicine researchers suggest that people can be infected again in the course of their life and thus contribute continuously infected feces to areas that lack water treatment facilities. In the United States, nearly all acute cases of hepatitis E occurred in travelers returning from countries where hepatitis E is endemic, such as India. However, there is evidence that between 1 and 5% of all healthy blood donors in the United States have HEV antibodies in the blood. The high rate of HEV antibodies has puzzled researchers from the CDC. Some suggest that high frequency of antibodies in the absence of symptoms and disease is due to infection with hepatitis E virus strain that causes the disease. It also suggests that animals may be acting as reservoirs of HEV and transferring a weakened form of virus to humans. For instance, in blood samples taken from 239 rats captured in Baltimore alleys, along the boardwalk Mississippi River in New Orleans, and urban and rural areas of Hawaii, tests revealed that over 80% of rats had HEV antibodies in the blood. Scientists have also identified a strain of HEV in pigs that is very similar to the strain that causes disease in humans. Among other major animal reservoirs of HEV antibodies are the sheep, cattle, and chickens. Tests and Diagnosis: When acute hepatitis E is symptomatic, there may be increases in liver enzymes, including alanine aminotransferase (ALT) and gamma glutamyl transpeptidase (GGT), which indicates inflammation or liver damage. When infected, the body's immune system first releases immunoglobulin M (IgM) antibodies to combat the foreign substance or antigen. IgM antibody levels decline rapidly three to six months after the outbreak of HEV infection. As a result, the amount of IgM antibodies in the blood allows the physician to diagnose what stage is an infection. Immunoglobulin (IgG) antibodies are also released to combat the virus. This type of antibody, which is the most abundant, can cross the blood vessel walls to combat the virus. It has been shown that IgG antibodies to hepatitis E persist from two to 13 years after the onset of infection. To diagnose hepatitis E in an infected patient, doctors measure the amount of HEV IgM antibodies or IgG antibodies in the blood of a patient. Currently there is only a market test to detect blood IgM and IgG antibodies to HEV. Now doctors are working to develop an ELISA test (enzyme-linked immunosorbent assay) for HEV IgM, enabling them to diagnose the disease in its early stages. There are other tests that can detect the RNA of hepatitis E in the blood and feces. However, the sensitivity of these tests are still being analyzed, and most are used primarily by institutions devoted to research. Vaccine and Treatment: Currently, there is no vaccine to prevent hepatitis E. Not even one prepared from immunoglobulin extracted from plasma of people infected with hepatitis E is effective in preventing the disease. Studies show that hepatitis E vaccines in animals reduce the infection of hepatitis E, but do not prevent elimination of the virus in the feces of infected immunized animals. So far, there is no treatment for hepatitis E. The only effective treatment is dealing with symptoms, not the disease. No antiviral treatment has proven effective against this virus in experiments. Preliminary studies in cell cultures suggest that ribavirin and interferon-α can inhibit the multiplication of hepatitis E. A possible target for medicines include the blocking of interactions of viral and cellular proteins that prevent virus replication. The only cure is prevention, which requires developing countries to purify drinking water and maintain water untreated sewage and water separate from drinking water sources. References: Jameel, S. (1999). Molecular biology and pathogenesis of hepatitis E virus. Expert Reviews in Molecular Medicine, http://www.ermm.cbcu.cam.ac.uk/99001271h.htm. Centers for Disease Control and Prevention, Hepatitis Division, Division of Viral and Rickettsial Diseases. Favorov, M.O., et al. (2000). Prevalence of Antibody to Hepatitis E Virus Among Rodents in the United States. The Journal of Infectious Diseases, 181: 449-455. Martins, E.B. (1998). Hepatitis Associated with Hepatitis A Superinfection in Patients with Chronic Hepatitis C. Letter to the Editor, The New England Journal of Medicine, 338:24. |