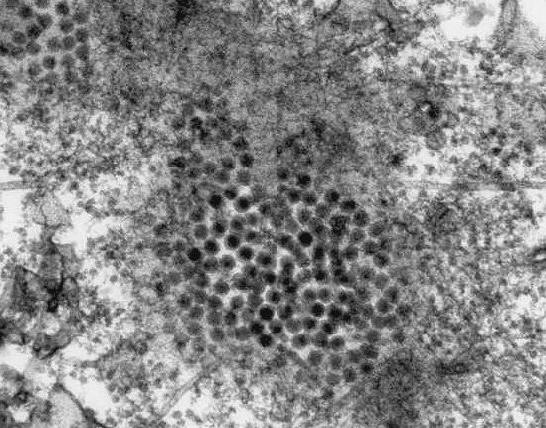
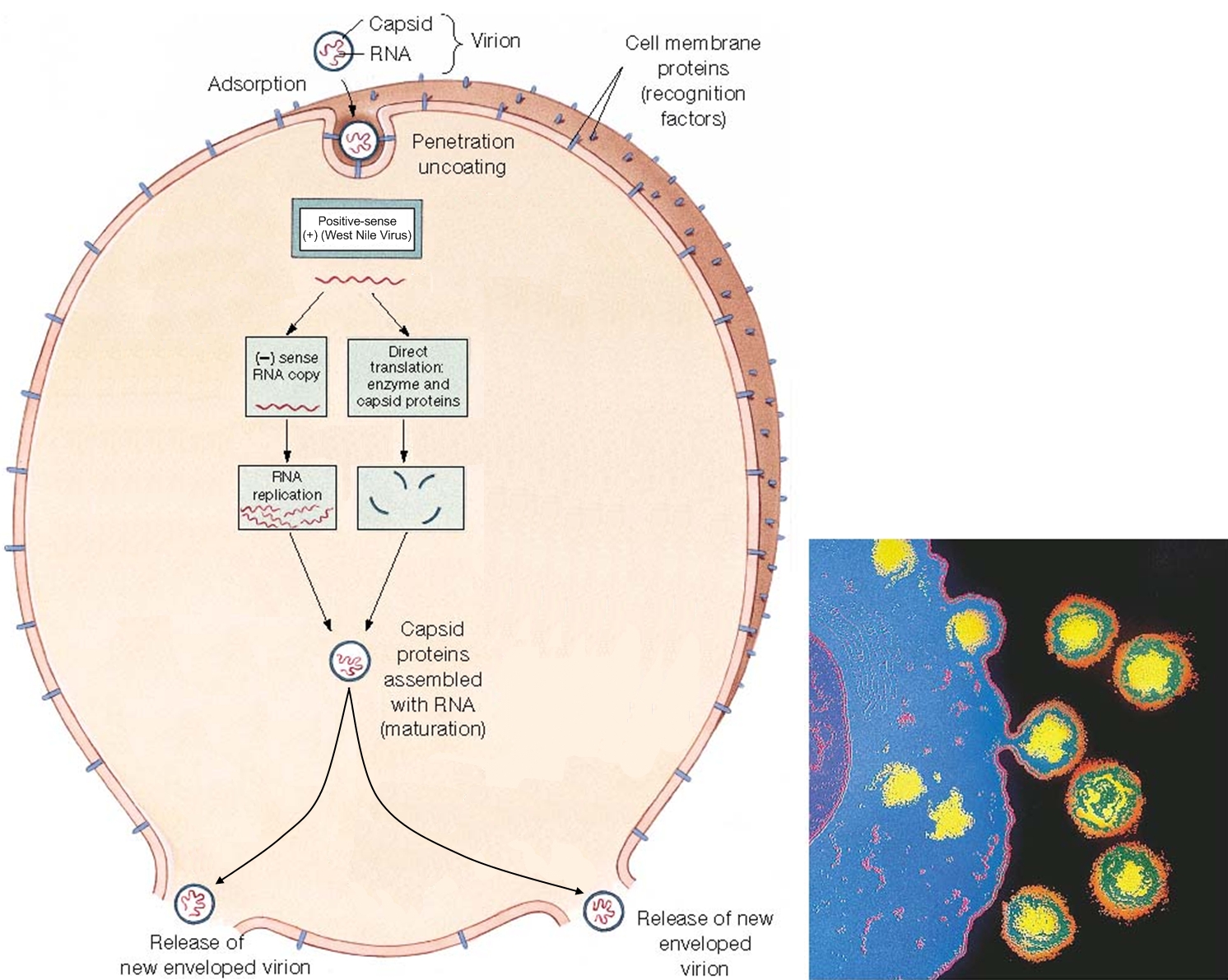
West Nile VirusOverview: West Nile virus is an enveloped, single-stranded, positive-sense RNA virus and a member of the genus Flavivirus and family Flaviviridae, which contains 70 recognized species (Figure 1). It has been reported that about 50% of these viruses are known to cause diseases in humans. In addition, these microorganism are arthropod-borne, and thus are referred to as 'arboviruses' (Figure 1). Arboviruses of the Flavivirus genus are capable of infecting and replicating in both vertebrate and invertebrate hosts. West Nile virus first appeared in North America in 1999 and rapidly spread across the continent, including parts of South America. It is commonly found in Africa, West Asia, and the Middle East and is closely related to St. Louis encephalitis virus found in the United States. Infection with West Nile virus causes West Nile fever, an influenza-like illness, usually self-limited, with an incubation period of 5 to 15 days, encephalitis, and West Nile meningitis. Figure 1. This transmission electron micrograph reveals the presence of West Nile virus virions, in an isolate that was grown in a cell culture. Life Cycle: The biological transmission cycle begins when a mosquito feeds on a West Nile virus-infected bird (Figure 2). The infected blood containing the virus spreads throughout the insect following its blood meal and replicates rapidly in its salivary glands. The mosquito can then transfer the virus to developing larvae growing near stagnant water or to vertebrates directly via a subsequent meal. Upon a second round of feeding, the mosquito successfully secretes saliva into its vertebrate host, thus transferring the virus to its new, more vulnerable end-host. In a very small number of cases, West Nile virus also has been spread through blood transfusions, organ transplants, breastfeeding, and even during pregnancy from mother to baby. The virus, however, is not spread through casual contact such as touching or kissing a person with the virus. Figure 2. The mosquito is a common known vector of West Nile virus. Pictured here (Ochlerotatus japonicus) is the mosquito is suspected of being a vector of the Japanese encephalitis virus in Asia, and the West Nile in North America. Pathogenicity: Once the virus' RNA (genetic material) is released into the cytoplasm of targeted cells, which include endothelium, lymphocytes (in particular, dendritic cells), and cells of the central nervous system, it acts as an early messenger RNA (mRNA) ready for translation of proteins responsible for the replicative processes (Figure 3). The strand of RNA is translated into a single polypeptide. The polypeptide is then cleaved into several proteins needed to construct the viral capsid. The capsid is enclosed in a lipid membrane that blocks premature viral fusion, and a glycosylated envelope (E) protein that mediates viral membrane fusion and attachment. The RNA also acts as a template for synthesis of minus (-) RNA (Figure 3). The minus (-) RNA subsequently acts as a template for new viral genomic RNA. Species containing an RNA-based genome often go unchecked in the cytoplasm because there are no particular host mechanisms capable of checking and correcting the replicated RNA; this typically results in mutations of the RNA product. The new virions are released from the infected cell and carried by the bloodstream to the lymph and central nervous system. Figure 3. A simplified illustration depicting the West Nile virus cellular life cycle. Click to enlarge. The feature that makes West Nile virus difficult to combat is its ability to cross the blood-brain barrier. When toll-like receptors (in particular, TLR-3) found on human immune cells detect patterns constituting the outer layer of the virus, the alerted cell activates an immune response that leads to the secretion of TNF-α. TNF-α temporarily breaks down or weakens the permeability of the blood-brain barrier during which it is proposed that the virus enters the central nervous system, and in turn, causes encephalitis and West Nile meningitis. TNF-α is typically released by T helper cells (TH17) as a proinflammatory cytokine that stimulates acute phase reactions. In order to evade the hosts immune response, West Nile virus uses many techniques to ward off human defenses. For instance, the virus has been shown to increase surface MHC class I molecule expression in vitro, which essentially prevents natural killer from detecting changes in an infected cell. Recall that natural killer cells recognize their target via activatory receptors known as killing-activated receptors (KAR) and inhibitory receptors. If a cell possess few MHC class I molecules on its surface, KAR receptors are activated to destroy the presumed infected cell. Furthermore, the West Nile virus is also capable of resisting the antiviral effects of interferons once it is successfully established inside the host. Recall that interferons, specifically IFN-γ, activate macrophages to destroy ingested pathogens via reactive oxygen species. However, both innate and adaptive immunity are required in collaboration to prevent early infection. Clinical Infections: Symptoms of West Nile infection include fever, encephalitis, hepatitis (liver inflammation), muscle weakness, shock syndrome, and cognitive impairment. According to studies performed on rodents, replication of the virus initially occurs in dendritic cells. Upon spreading to the lymph nodes and infecting peripheral tissues of the spleen and kidneys, the virus typically manifests in the central nervous system by the first week of infection. West Nile virus infection symptoms are usually very mild. It is noted that approximately 80% of individuals infected show no symptoms whatsoever. Complete recovery results in healthy, normal persons, whereas those who show prolonged effects usually have on-going muscle weakness, fatigue, and even paralysis in the most extreme cases. Scientists and researchers do not know why some individuals recover while others succumb to infection. Nonetheless further research is needed to be conducted to understand the West Nile virus and host interaction. In addition, there is also a need to understand exactly how this virus is able to pass the blood-brain barrier and directly infects the central nervous system of its host. Presently, proposed vaccines for animals exist, but detailed investigation based on the immunologic response to the infection are needed to help design new vaccines. References: Calisher, C.H. (2007). Arthropod Borne Viruses. Encyclopedia of Life Sciences, 7:1-9. Samuel, M.A., & Diamond, M.S. (2006). Pathogenesis of West Nile Virus Infection: a Balance between Virulence, Innate and Adaptive Immunity, and Viral Evasion. Journal of Virology, 80(19): 9349-9360. |