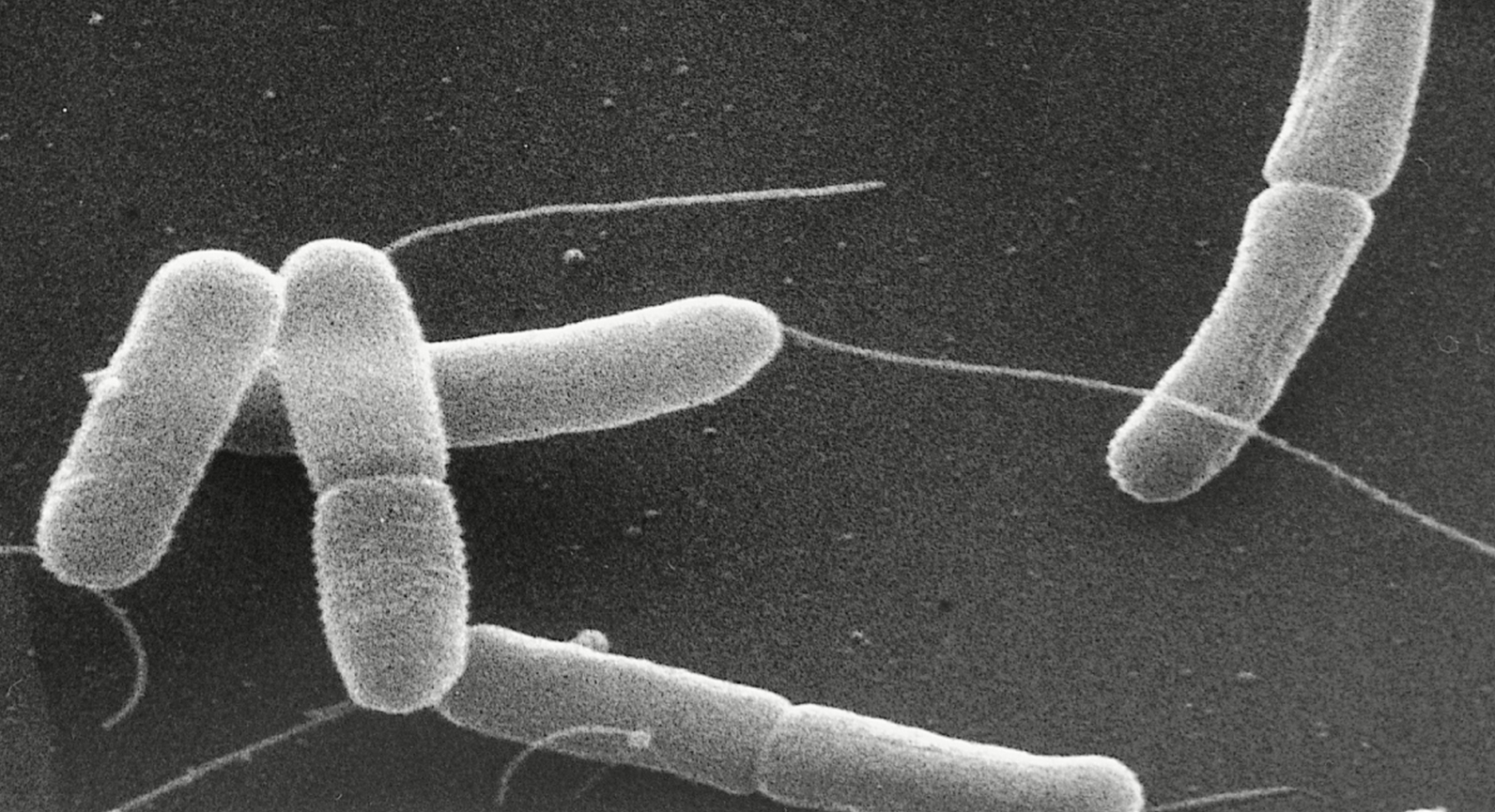
Enterotoxigenic Escherichia coliOverview: Enterotoxigenic Escherichia coli (ETEC) is a Gram-negative, rod-shaped bacterium that causes traveler's diarrhea (Figure 1). ETEC bacteria are motile facultative anaerobes (they can makes ATP energy by aerobic respiration if oxygen is present, but is also capable of switching to fermentation) that can easily be cultured under aerobic conditions at 37°C. Normally, E. coli serves a useful function in the body by suppressing the growth of harmful bacterial species in the small intestine, and by synthesizing appreciable amounts of vitamins. However, this particular strain leads to a major cause of diarrheal disease in underdeveloped nations that lack sanitation infrastructure, especially among children (Compare with other strains). What differentiates the various strains of E. coli is when the bacteria carries an extra piece of genetic information encoded on a plasmid that, when translated into a protein, produces toxic effects on the host. The toxins and the diseases that ETEC causes are not related to E. coli O157:H7. Figure 1. This scanning electron micrograph reveals some of the morphologic details displayed by two joined Gram-negative Enterotoxigenic Escherichia coli (0:169 H41) bacteria dividing by binary fission [22409 X]. Mechanism of Infection: ETEC is able to infect its host using a number of virulence factors, including adhesion proteins (fimbrial adhesins) that adhesins mediate the attachment of bacteria to the surface of host epithelium cells and allow bacterial colonization, and the production of toxins (Zhang et al., 2006). In pigs, ETEC strains that produce adhensins are the most common currently associated with diarrheal diseases. These fimbriae bind to glycoconjugates on enterocytes (intestinal cells), and the absence of the respective glycoconjugate renders the animal resistant to bacterial colonization and consequent diarrheal diseases. ETEC produce one or both of two potent enterotoxins, namely, heat-labile (LT) and heat-stable (ST) toxin. Both toxins, specifically LT, are similar in structure and function to cholera toxin. As such, they have been found to disrupt intestinal fluid homeostasis and to cause hypersecretion of fluid and electrolytes through activation of adenylate cyclase (by LT) or guanylate cyclase (by ST) in small intestinal mucosal cells (Zhang et al., 2006). This mechanism elevates either cyclic AMP or cyclic GMP levels in intestinal epithelial cells, stimulate active chloride secretion, inhibit electroneutral sodium chloride absorption in the intestinal epithelium, and subsequently cause unidirectional fluid secretion (diarrhea). LT is highly immunogenic, but lacks toxicity. Consequently, it is used in current vaccines against ETEC. Heat-stable toxin (ST) is a small peptide that causes secretion of fluids and electrolytes, but it lacks immunogenicity (Rosales-Mendoza et al., 2009). Between the two, the effect of LT on the host is substantially greater than that of ST (Zhang et al., 2006). Treatment: Most infections caused by ETEC bacteria are self-limiting and do not require anti-microbial treatment due to the short duration of symptoms; thus, the infection will end on its own and is rarely life-threatening. Most cases of the infection arise from contamination of water used for drinking and food preparation with fecal matter. Developed countries with proper sanitation have a much lower incidence of ETEC infection (Devasia et al., 2006). A lack of proper food sanitation techniques and allowing a meal to remain unrefrigerated for hours allows what may have been a insignificant amount of contamination to multiply to dangerous levels. Treatment of an ETEC infection is limited to supportive care and, more importantly, oral rehydration to replace fluids lost from diarrhea. Identification:
Identification of ETEC from a
stool sample culture is difficult, since it cannot be distinguished from
E. coli
that is native to the gastrointestinal
tract. When stool
cultures do not show a routine enteric pathogen,
symptoms may be attributed
to a viral cause without confirmatory tests
(Dalton et
al., 1999). Because further
testing would extend after symptoms have
subsided, it is not usually pursued.
Biochemical and serotyping methods are typically
used for identification of References: Dalton, C.B., Mintz, E.D., Wells, J.G., Bopp, C.A. & Tauxe, R.V. (1999). Outbreaks of enterotoxigenic Escherichia coli infection in America adults: a clinical and epidemiologic profile. Epidemiology and Infection, 123: 9-16. Devasia, R.A., Jones, T.F., Ward, J., Stafford, L., Hardin, H., Bopp, C., Beatty, M., Mintz, E. & Schaffner, W. (2006). Endemically Acquired Foodborne Outbreak of Enterotoxin-producing Escherichia coli Serotype O169:H41. The American Journal of Medicine, 119: 6-10. Rosales-Mendoza, S., Alpuche-Solis, A.G., Soria-Guerra, R.E., Moreno-Fierros, L., Martinez-Gonzalez, L., Herrera-Diaz, A. & Korban, S.S. (2009). Expression of an Escherichia coli antigenic fusion protein comprising the heat labile toxin B subunit and the heat stable toxin, and its assembly as a functional oligomer in transplastomic tobacco plants. The Plant Journal, 57: 45-54. Stenutz, R., Weintraub, A. and Widmalm, G.
(2006). The
structures of Escherichia coli Opolysaccharide Zhang, E.M., Berberov, J.F., et al. (2006). Significance of Heat-Stable and Heat-Labile Enterotoxins in Porcine Colibacillosis in an Additive Model for Pathogenicity Studies. Infection and Immunity, 74(6): 3107-3114. |